| |
Regimen Simplification to Atazanavir-Ritonavir Alone as Maintenance Antiretroviral Therapy After Sustained Virologic Suppression
|
| |
| |
JAMA. 2006;296:806-814.
Vol. 296 No. 7, August 16, 2006
Susan Swindells, MBBS; A. Gregory DiRienzo, PhD; Timothy Wilkin, MD, MPH; Courtney V. Fletcher, PharmD; David M. Margolis, MD; Gary D. Thal, MD; Catherine Godfrey, MD; Barbara Bastow, RN, BSN; M. Graham Ray, MSN; Hongying Wang, MS; Robert W. Coombs, MD, PhD; John McKinnon, MD, MSc; John W. Mellors, MD; for the AIDS Clinical Trials Group 5201 Study Team
"....In this pilot study, the data suggest that simplified maintenance therapy with atazanavir-ritonavir alone in patients who have never experienced treatment failure may be efficacious in maintaining HIV-1 RNA suppression below 200 copies/mL for 24 weeks after discontinuing NRTIs. Among the 31 participants in whom HIV-1 RNA remained suppressed, almost all measurements (97%) below 200 copies/mL were also below 50 copies/mL and consecutive values above 50 copies/mL were not observed. Virologic failure occurred in 3 participants (9%) and was associated with low or undetectable plasma atazanavir concentrations in 2 participants. Importantly, virologic failure was not associated with the development of PI resistance mutations, and viremia resuppressed to below 50 copies/mL in all participants who experienced failure....Although the findings herein concerning the potential benefit of simplified maintenance therapy with atazanavir-ritonavir are encouraging, caution regarding inferences is warranted due to study limitations such as the small number of participants represented in the analysis, the use of a .10 level and 1-sided testing, and the few individuals discontinuing the study and those experiencing virologic failure....." note from Jules Levin: since this publication results from 3 larger Kaletra monotherapy studies were reported at IAC Toronto, Aug 2006. You can read these study results on the NATAP website at http://www.natap.org in the Conference Reports section for IAC Toronto 2006.
ABSTRACT
Context The long-term adverse effects, expense, and difficulty of adherence to antiretroviral regimens have led to studies of simpler maintenance therapies. Maintenance therapy with ritonavir-boosted atazanavir alone is a possible option because of low pill burden, once-daily dosing, safety, and unique resistance profile.
Objective To assess whether simplified maintenance therapy with atazanavir-ritonavir alone after virologic suppression increases the risk of virologic failure (2 consecutive human immunodeficiency virus type 1 [HIV-1] RNA measurements of 200 copies/mL).
Design, Setting, and Participants Single-group, open-label, multicenter, 24-week pilot study of 36 HIV-infected adults with virologic suppression for 48 weeks or longer receiving their first protease inhibitor (PI)-based regimen. The study was conducted between September 1, 2004, and April 18, 2006, at 12 participating AIDS clinical trial units in the United States.
Intervention Participants switched PIs to atazanavir-ritonavir at entry and discontinued nucleoside analog reverse transcriptase inhibitors (NRTIs) after 6 weeks.
Main Outcome Measures Virologic failure within 24 weeks of discontinuing NRTIs. Other measures included HIV-1 drug resistance, plasma atazanavir concentrations, adverse events, CD4 cell counts, plasma lipid levels, and HIV-1 RNA levels in seminal plasma.
Results Thirty-six participants enrolled and 2 discontinued before simplification to atazanavir-ritonavir alone. Thirty-four patients were included in the analysis of the primary end point after 24 weeks: 1 withdrew voluntarily, and 33 continued the regimen. Virologic success (absence of failure) through 24 weeks of simplified therapy occurred in 91% (31 of 34 patients; lower 90% confidence interval limit = 85%). Three participants experienced virologic failure 12, 14, and 20 weeks after simplification, with plasma HIV-1 RNA levels of 4730, 1285, and 28 397 copies/mL, respectively. Resistance testing at failure did not identify PI resistance mutations. Plasma atazanavir concentrations at failure were low or below detection in 2 of 3 participants experiencing failure. There were no treatment discontinuations for adverse events after simplification; no significant changes in CD4 cell counts or plasma lipid levels; and no detectable HIV-1 RNA in seminal plasma from all 8 participants tested.
Conclusions These preliminary data suggest that simplified maintenance therapy with atazanavir-ritonavir alone may be efficacious for maintaining virologic suppression in carefully selected patients with HIV infection. These findings require confirmation in larger, randomized trials of this strategy.
INTRODUCTION
The long-term adverse effects, expense, and inherent difficulty of sustained adherence to multidrug antiretroviral regimens have prompted studies of simpler therapies for human immunodeficiency virus type 1 (HIV-1) infection. Treatment cessation, intermittent therapy, and induction-maintenance regimens have been evaluated with mostly inferior results. A large clinical trial comparing continuous and CD4 cell count-guided intermittent therapy was recently discontinued after an interim analysis found twice the risk of disease progression and death with intermittent therapy.1 Shorter cycles of intermittent therapy showed promise in a pilot trial,2 but a larger study was discontinued for significantly greater risk of virologic failure.3
Earlier trials of induction therapy with standard regimens, followed by simplified maintenance with 1 or 2 drugs, were also discontinued because of significantly greater risk of virologic failure in the maintenance phase.4-6 More recent pilot studies of maintenance therapy with a single protease inhibitor (PI) "boosted" with ritonavir to increase drug exposure have provided promising results.7-9
Simplified maintenance therapy with a single boosted PI offers the potential advantages of decreased risk or reversal of toxicities associated with nucleoside analog reverse transcriptase inhibitor (NRTI) use such as mitochondrial dysfunction, hyperlactatemia, lactic acidosis, lipoatrophy, and peripheral neuropathy.10-11 This strategy also has the potential to reduce the cost of PI-based antiretroviral therapy.
Atazanavir with ritonavir (atazanavir-ritonavir) is a possible option for maintenance therapy for several reasons. A randomized trial of 200 treatment-naive patients comparing atazanavir with and without ritonavir in combination with lamivudine and extended-release stavudine found virologic responses (<50 copies/mL) in 75% vs 70%, respectively, over 48 weeks.12 In treatment-experienced patients, atazanavir-ritonavir combined with 2 NRTIs showed viral suppression similar to lopinavir-ritonavir (38% vs 46% with plasma HIV-1 RNA levels <50 copies/mL at week 48).13 Atazanavir is a well-tolerated PI with lower likelihood of plasma lipid elevations compared with other drugs in this class.14-15 Virologic failure with atazanavir as initial therapy is associated with a distinct resistance profile and absence of PI cross-resistance, suggesting that multiple second-line treatment options are available in this event.
These characteristics make ritonavir-boosted atazanavir potentially useful for simplified maintenance therapy, but the strategy has not been rigorously evaluated in a clinical trial. This study was designed and conducted to test the hypothesis that simplified maintenance therapy with atazanavir-ritonavir alone after virologic suppression does not markedly increase the risk of virologic failure.
RESULTS
Study Participants
Thirty-six participants were enrolled between September 1, 2004, and April 25, 2005. Week 24 data were analyzed between December 5, 2005, and April 18, 2006. Baseline characteristics are given in Table 1. The median age was 40 years; 92% of patients were male, 61% were white, 25% were black, and 11% were Hispanic. The median CD4 cell count at entry was 616 cells/mm3, and the median nadir CD4 cell count was 253 cells/mm3.
Two participants discontinued the study (see Methods) after switching to atazanavir-ritonavir but before simplification to atazanavir-ritonavir alone. Of the 34 patients who proceeded to simplified maintenance therapy, none discontinued study treatment because of adverse events. One patient voluntarily withdrew from the study because of distance from the clinic and demands of full-time employment; all other participants remained in the study through 24 weeks after simplification.
Virologic Outcomes
Observed virologic success through 24 weeks after simplification was 91% (31 of 34 participants; lower 90% CI limit = 85%). HIV-1 RNA measurements were available for 224 (94%) of the 239 expected measurements. Among the 31 virologic successes, the HIV-1 RNA measurement was below 50 copies/mL in 199 (97%) of 206 samples tested through 24 weeks, and consecutive HIV-1 RNA levels of 50 copies/mL or higher were not observed.
Three patients met criteria for virologic failure (2 consecutive values of 200 copies/mL) at weeks 12, 14, and 20, with the first plasma HIV-1 RNA level above 200 copies/mL being 4730, 1285, and 28 397 copies/mL, respectively. Genotyping of plasma samples from the 3 patients experiencing virologic failure did not identify drug resistance mutations in protease. One patient had the L63P polymorphism in protease codon, but a pretherapy genotype was not available for comparison. The L63P polymorphism is not considered a resistance mutation because 63P is a frequent polymorphism in sequences from untreated patients in the Los Alamos database.22 The K103N reverse transcriptase resistance mutation was detected in the same sample, but the patient had no history of exposure to NNRTIs.
Of the 3 participants experiencing virologic failure, one continued taking atazanavir-ritonavir alone and the plasma HIV-1 RNA value resuppressed to below 50 copies/mL by week 20 (6 weeks after virologic failure). A second patient changed therapy to emtricitabine-tenofovir with lopinavir-ritonavir and subsequently missed some study visits but had a plasma RNA level of below 50 copies/mL at week 32 (20 weeks after virologic failure). The third patient changed therapy to emtricitabine-tenofovir with continued atazanavir-ritonavir 6 weeks after virologic failure, but missed 2 subsequent study visits because of interval development of depression. The plasma HIV-1 RNA level 6 weeks after restarting combination therapy was 1893 copies/mL and below 50 copies/mL at week 41 (21 weeks after virologic failure).
Pharmacokinetic Analyses and Medication Adherence
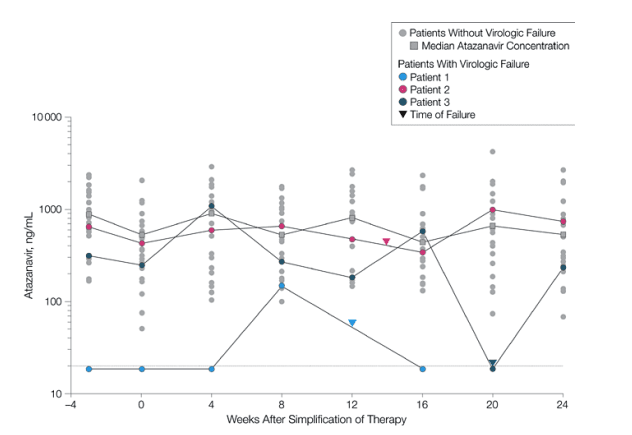
Table 2 shows atazanavir pharmacokinetic adherence information for all 36 patients and data for those who did and did not experience virologic failure. The 3 patients who had virologic failure had a greater frequency of study visits when no atazanavir could be detected in their plasma, and therefore a significantly lower median atazanavir exposure for their duration of study treatment, compared with patients who did not have virologic failure (P<.001).
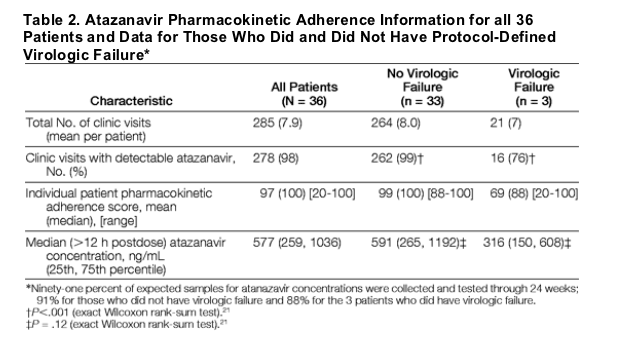
The Figure shows the median atazanavir concentration in nanograms per milliliter by study visit for the patients who did not have virologic failure and the individual atazanavir concentrations for the 3 patients who did. The first patient whose atazanavir-ritonavir maintenance therapy failed had undetectable atazanavir concentrations for all except the week 8 visit, and virologic failure occurred at week 12. The second participant had drug concentrations similar to the group median throughout the study. This individual continued taking atazanavir-ritonavir alone after virologic failure and resuppressed to an HIV-1 RNA level below 50 copies/mL. This suggests the possibility of a period of suboptimal drug exposure; ie, even though the drug concentrations at study visits and at virologic failure were similar to the group median, this does not exclude a potential period of nonadherence. The third participant maintained adequate atazanavir concentrations through week 16. At week 20, atazanavir was undetectable in plasma and virologic failure occurred.
Figure. Atazanavir Concentrations Over Time in Patients With and Without Virologic Failure
Median concentration levels for patients without virologic failure are shown as ng/mL. Atazanavir concentrations in the 3 patients who experienced failure are indicated with numbers 1, 2, and 3; times of virologic failure are indicated with circles. The lower limit of quantitation for the atazanavir assay is 20 ng/mL (dotted line).
Self-reported adherence data were collected from all study participants. One of the 3 participants with virologic failure reported never skipping medications (the patient whose therapy failed at week 14); 1 patient reported missing 2 to 4 weeks ago (the patient whose therapy failed at week 12); and 1 patient reported missing within the last week (the patient whose therapy failed at week 20). These reports of nonadherence were not quantitatively different from the 33 participants without virologic failure. Of the 33 patients not having virologic failure, 18 reported never skipping medications; 4 reported skipping more than 3 months ago; 5 reported skipping 1 to 3 months ago; 5 reported missing 1 to 2 weeks ago; and 1 reported skipping in the past week.
HIV-1 RNA in Seminal Plasma
Eight participants provided semen samples 24 weeks after simplification of therapy. HIV-1 RNA in seminal plasma was below the limit of detection (150 copies/mL) in all 8 specimens.
Other Study Outcomes
Neither absolute CD4 cell counts nor percentages changed significantly during the course of the study. The median CD4 cell count at simplification was 588/mL and 604/mL after 24 weeks (26 participants contributed to both time points).
Of the 24 patients who had measurements at baseline and after 24 weeks, there were no significant changes in fasting cholesterol or triglyceride values, although baseline levels were not elevated and values were not censored for those receiving lipid-lowering therapy. The mean change over 24 weeks was as follows: total cholesterol, -4.5 mg/dL (-0.12 mmol/L) (P = .53); high-density lipoprotein cholesterol, 4.33 mg/dL (0.11 mmol/L) (P = .11); low-density lipoprotein cholesterol, -5.2 mg/dL (-0.31 mmol/L) (P = .51); and triglycerides, -18.17 mg/dL (-0.21 mmol/L) (P = .40).
One patient reported grade 3 symptoms (aches, pain, discomfort), but there were no other grade 3 or 4 signs or symptoms reported. During the study there were 12 grade 3 and 6 grade 4 laboratory toxicities (18 in all in 17 patients), 17 of which were unconjugated hyperbilirubinemia, a recognized adverse effect of atazanavir. No AIDS-defining events occurred, and no patients discontinued the study medication during this phase because of adverse events.
COMMENT
In this pilot study, the data suggest that simplified maintenance therapy with atazanavir-ritonavir alone in patients who have never experienced treatment failure may be efficacious in maintaining HIV-1 RNA suppression below 200 copies/mL for 24 weeks after discontinuing NRTIs. Among the 31 participants in whom HIV-1 RNA remained suppressed, almost all measurements (97%) below 200 copies/mL were also below 50 copies/mL and consecutive values above 50 copies/mL were not observed. Virologic failure occurred in 3 participants (9%) and was associated with low or undetectable plasma atazanavir concentrations in 2 participants. Importantly, virologic failure was not associated with the development of PI resistance mutations, and viremia resuppressed to below 50 copies/mL in all participants who experienced failure.
Although earlier clinical trials of induction-maintenance strategies yielded mixed results, more recent studies of ritonavir-boosted PI therapy, including this one, have shown promise. In a pilot trial of 12 patients who simplified therapy to indinavir with ritonavir alone after prolonged HIV-1 RNA suppression with standard therapy, virologic failure did not occur over a median follow-up of 78 weeks.7 However, renal toxicity in 4 of the participants suggests that indinavir is not the optimal agent for this strategy. The "Only Kaletra" study was a randomized comparison of standard of care therapy with 2 NRTIs and ritonavir-boosted lopinavir vs simplified maintenance therapy with lopinavir-ritonavir alone.8 By intent-to-treat analysis, HIV-1 RNA levels remained suppressed to below 50 copies/mL in 17 (81%) of 21 participants randomized to receive lopinavir-ritonavir alone at 48 weeks compared with 20 (95%) of 21 participants in the standard of care arm. In the lopinavir-ritonavir alone arm, there were 3 virologic failures (confirmed viral load >500 copies/mL) and 1 patient was lost to follow-up. In the standard of care arm, 1 patient discontinued the study because of dyslipidemia.
In another observational study of ritonavir-boosted atazanavir as maintenance therapy, 2 of 28 patients experienced virologic failure during 24 weeks of follow-up.9 Nine patients in this cohort also participated in the indinavir study cited above.7 As in our study, participants in these studies who experienced virologic failure did not develop PI resistance. Taken together, these data suggest that simplified therapy with a boosted PI alone may be efficacious for maintenance therapy in patients with no prior history of treatment failure or drug resistance and that failure of simplified therapy is not associated with the emergence of PI resistance. However, the findings represent a preliminary assessment of the potential benefit of simplified maintenance therapy with atazanavir-ritonavir, and safety and efficacy cannot be definitively demonstrated with the small numbers of study participants represented herein.
Concern exists that the use of PIs alone will not provide adequate drug penetration into anatomic compartments such as the central nervous system or the genital tract. In addition to possible increased risk of virologic breakthrough, detectable HIV-1 RNA in the genital tract could increase the likelihood of sexual transmission. Although combination antiretroviral therapy reduces levels of HIV-1 RNA in cerebrospinal fluid (CSF) and semen, virus may still be detected in CSF in 1% of patients with plasma HIV-1 RNA levels below 50 copies/mL (David B. Clifford, MD, Washington University School of Medicine, oral communication, November 14, 2005).
Moreover, approximately 2% of men with plasma HIV-1 RNA measurements below 400 copies/mL have detectable HIV-1 RNA in seminal plasma.23-24 Atazanavir penetrates into seminal fluid and CSF, but a longitudinal evaluation of anti-HIV activity in these compartments has not been done.25 In our study, HIV-1 RNA in seminal plasma was below the limit of detection in all 8 specimens 24 weeks after simplification of therapy to atazanavir-ritonavir alone. Careful studies of HIV-1 RNA levels in semen and in CSF after simplification of therapy to atazanavir-ritonavir alone are needed, particularly since atazanavir concentrations are often low or undetectable in CSF.26
Measured concentrations of drugs provide proof of drug exposure; however, they have not been widely used as a measure of adherence. This stems from drug measurements not being widely available, and experience with early antiretroviral agents, such as NRTIs, which have short elimination half-lives and undetectable drug concentration throughout the dosing interval even among adherent persons. The availability and use of antiretroviral agents like efavirenz and ritonavir-boosted PIs that have long elimination half-lives means that with modern analytical techniques, concentrations are always detectable at any point in a dosing interval in adherent patients. With regard to ritonavir-boosted atazanavir, the geometric mean 24-hour postdose concentration following 300 mg of atazanavir with 100 mg of ritonavir is 636 ng/mL. The analytical procedure used in this study could quantify concentrations down to 20 ng/mL; therefore, the inability to measure any atazanavir in the plasma of a patient is suggestive of nonadherence as the cause of undetectable atazanavir.
The frequency of study visits with undetectable atazanavir concentrations was significantly higher among participants experiencing virologic failure compared with those whose HIV-1 RNA levels remained suppressed (Table 2; P<.001), indicating that intermittent medication nonadherence was the likely cause of virologic failure. Indeed, virologic failure was temporally associated with undetectable atazanavir concentrations in 2 of 3 participants (Figure). Furthermore, the participants with virologic failure were not taking concomitant medication that could lower atazanavir concentrations, and these would not be expected to reduce concentrations to undetectable.
Drug concentrations have been used in investigations of other antiretroviral agents as a metric of adherence. The ACTG 359 trial was a factorial study of 6 antiretroviral regimens, all including saquinavir, among 258 HIV-infected persons whose prior therapy had failed. In this study, 4 measures of adherence were evaluated: counts of returned medications, self-reported adherence, electronic monitoring of the opening of medication vials, and measured saquinavir concentrations. Only self-reported adherence and measured saquinavir concentrations were significant predictors of virologic response, supporting the value of drug concentrations as an indicator of adherence.27
Although the findings herein concerning the potential benefit of simplified maintenance therapy with atazanavir-ritonavir are encouraging, caution regarding inferences is warranted due to study limitations such as the small number of participants represented in the analysis, the use of a .10 level and 1-sided testing, and the few individuals discontinuing the study and those experiencing virologic failure.
CONCLUSIONS
Maintenance therapy with a single boosted PI offers a treatment strategy with potentially less complexity, pill burden, long-term complications, and cost. Atazanavir is well tolerated and associated with decreased risk of dyslipidemia, compared with other PIs.14-15 Virologic failure with atazanavir as initial therapy is associated with a distinct resistance profile and absence of PI cross-resistance, suggesting that multiple second-line treatment options are available in this event. These characteristics make boosted atazanavir a promising candidate for maintenance therapy, and the preliminary results presented herein support this strategy. Larger, randomized trials comparing this approach with standard antiretroviral therapy are warranted.
METHODS
Study Design and Participants
The AIDS Clinical Trials Group (ACTG) 5201 study was a prospective, open-label pilot trial of regimen simplification to atazanavir-ritonavir alone after sustained virologic suppression. Participants were recruited between September 1, 2004, and April 25, 2005. The 24-week data were analyzed between December 5, 2005, and April 18, 2006. Data collection for the follow-up to 48 weeks is ongoing.
The primary objective was to evaluate the risk of virologic failure (defined as 2 consecutive plasma HIV-1 RNA levels of >200 copies/mL) in patients through 24 weeks after simplification of therapy to atazanavir-ritonavir alone. Secondary objectives were (1) to evaluate the safety and tolerability of the treatment by clinical assessments and laboratory monitoring; (2) to test for PI-resistant variants in plasma samples at virologic failure; (3) to evaluate change in plasma lipid levels after regimen simplification; (4) to evaluate changes in absolute and percentage CD4 cell counts after simplification; (5) to investigate relationships among self-reported adherence assessed by a validated questionnaire developed by the ACTG,16 measured plasma atazanavir concentrations, and time to virologic failure; and (6) to estimate the prevalence of detectable HIV-1 RNA in genital secretions 24 weeks after treatment with atazanavir-ritonavir alone.
Patients were recruited from 12 clinical trials units of the Adult ACTG by investigators at the clinical sites. In keeping with site institutional review board guidelines, some participants were reimbursed for travel and child care expenses, but otherwise were not paid as volunteers. Inclusion criteria were as follows: men and nonpregnant women aged 18 years or older; laboratory documentation of HIV-1 infection; receiving first potent antiretroviral regimen, defined as at least 2 NRTIs plus at least 1 PI, for at least 48 weeks immediately prior to entry; CD4 cell count of 250/mL or higher; plasma HIV-1 RNA level below 50 copies/mL for at least 48 weeks prior to entry; having certain laboratory parameters (absolute neutrophil count >750/mL [>750 x 109/L]; hemoglobin >7.0 g/dL [>70 g/L]; platelet count >50 000/mL [>50 000 x 109/L]; serum creatinine 3 times upper limit of normal; aspartate and alanine aminotransferase and alkaline phosphatase levels 5 times upper limit of normal; total bilirubin 5.0 times upper limit of normal and conjugated bilirubin <2.5 times upper limit of normal; and serum lipase <1.5 times upper limit of normal). Race and ethnicity assignments were made by self-report and not determined by the investigator.
Patients were required to be naive to treatment with nonnucleoside reverse transcriptase inhibitors (NNRTIs), and patients with previously documented HIV-1 resistance to PIs were excluded. Patients with positive hepatitis B surface antigen also were excluded because of discontinuing NRTIs that may have been required for treatment of hepatitis B coinfection. Patients with preexisting cardiac conduction system disease were also excluded because of initial reports of abnormalities of atrioventricular conduction, since discounted, leading to concern that atazanavir might prolong the PR interval of the electrocardiogram in some patients.17
Institutional review boards of the participating institutions unconditionally approved the study, and each patient gave written informed consent. Reference was made to publication of data and protection of confidentiality by the statement in the informed consent document indicating that "any publication of this study will not use your name or identify you personally."
Study Treatment
At entry, patients discontinued their current PIs and began taking atazanavir (300 mg, once daily [two 150-mg capsules]) with ritonavir (100 mg, once daily) for 6 weeks. Patients with plasma HIV-1 RNA levels of 50 copies/mL or higher after 3 weeks and/or those who experienced treatment-limiting adverse effects during the 6-week lead-in were discontinued from the study and replaced. One patient voluntarily withdrew because of scleral icterus, and a second had a plasma HIV-1 RNA level of 50 copies/mL 3 weeks after switching to atazanavir-ritonavir; these 2 individuals were replaced. Otherwise, patients discontinued NRTIs after 6 weeks and continued taking atazanavir-ritonavir alone for the next 24 weeks, with continued follow-up planned for 48 weeks after simplification (ongoing).
Follow-up is ongoing for safety and efficacy of use of atazanavir-ritonavir alone. However, the primary end point of this trial was 24 weeks after discontinuing NRTIs, and this is the end point reported herein. There were no treatment-limiting adverse events after discontinuing NRTIs. Use of proton pump inhibitors was not allowed during the study because they are known to lower atazanavir concentrations.14
Study Monitoring
Patients were scheduled for follow-up visits every 4 weeks after simplification. Follow-up consisted of clinical assessments, routine safety laboratory monitoring using the Division of AIDS table for grading the severity of adverse events,18 and HIV-1 RNA measurement. Patients' CD4 cell counts were measured every 8 weeks; fasting lipid levels were measured at baseline and 24 weeks. Self-reported adherence was assessed by a validated questionnaire developed by the ACTG16 at baseline and study weeks 4, 12, and 24; the questions used involved time since last dose of medication was missed, measured in time increments over the previous 3 months. A safety monitoring committee examined the study results when 16 patients had been enrolled and recommended continuing the study unchanged.
Plasma samples for quantitation of atazanavir concentration were obtained every 4 weeks for the first 24 weeks and then at 8-week intervals. Research staffs were requested to obtain the plasma sample for atazanavir concentration as consistently as possible after dosing and between 12 to 24 hours after the last dose of atazanavir. A reversed-phase high performance liquid chromatographic (HPLC) assay for the determination of atazanavir concentrations was developed and validated. The HPLC system consisted of a separations module with a dual wavelength ultraviolet absorbance detector (Waters Alliance 2690 module and Waters 2487 absorbance detector; Waters Corp, Milford, Mass). The chromatographic separation of the compounds and the internal standard was accomplished on a YMC, C8 column (100 x 4.6 mm, S-3 micron 120A; Waters Corp). The mobile phase consisted of 54.7% 20-mM acetate buffer/45.3% acetonitrile, pH 4.9 with an isocratic flow rate of 1 mL/min. Detection and quantitation of the drugs was at 212 nm. A liquid-liquid extraction procedure with t-butylmethylether at basic pH was used to prepare the samples. All chemicals were purchased from Fisher Scientific, Fairlawn, NJ. The assay uses 0.2 mL of EDTA plasma and has a 20 ng/mL limit of quantitation (LOQ) for all analytes tested. The assay standard curve and linearity range is from 20 ng/mL to 20 000 ng/mL (upper LOQ). Inter- and intraday accuracy and precision are within ±20% at the LOQ and ±15% at all other concentrations. The HIV-1 RNA levels were measured in the seminal plasma of a subset of patients after 24 weeks of taking atazanavir-ritonavir alone as previously described.19 All participants were invited to undergo genital secretion sampling, and 8 males volunteered.
Patients who had HIV-1 RNA levels of 200 copies/mL or higher were asked to return to the clinic within 30 days for repeat measurement to confirm (or exclude) virologic failure. Real-time genotyping (ViroSeq version 2.6; ABI, Foster City, Calif) was performed if the second HIV-1 RNA measurement was 500 copies/mL or higher. All changes in protease (codons 1-99) and reverse transcriptase (codons 1-335) from HIV-1 subtype B consensus are reported. Results were provided to the site investigators and decisions about subsequent antiretroviral therapy were made by the site investigators and/or primary care clinicians. Three patients experienced virologic failure and genotyping was performed. No drug resistance mutations in protease were observed, and treatment changes were not based on genotype. All patients who permanently discontinued the study treatment, including those who changed therapy because of virologic failure, were followed up.
Statistical Considerations
The primary study end point was virologic failure, defined as 2 consecutive plasma HIV-1 RNA measurements of 200 copies/mL or higher at or before 24 weeks while taking atazanavir-ritonavir alone. A Kaplan-Meier estimate of the distribution of the time from simplification to virologic failure was calculated and the value at 24 weeks obtained, giving an estimate of the probability of no virologic failure (ie, virologic success) from simplification through 24 weeks while taking atazanavir-ritonavir alone.
The time to censoring was defined as the time from simplification to the last study visit at or before dropout or the last study visit at or before the time of analysis, whichever came first. This censoring time corresponds to an intent-to-treat primary analysis. The analysis was preplanned. Missing HIV-1 RNA measurements were not treated as failures; HIV-1 RNA measurements were available for 224 (94%) of the 239 expected measurements. Using the Greenwood variance formula, a 90% 1-sided confidence interval (CI) (only the lower limit was specified) was constructed for the probability of virologic success 24 weeks after simplification to atazanavir-ritonavir alone:
Let S(u) denote the Kaplan-Meier estimate at time u. Let t(1), . . . ,t(k) be the distinct observed event times at or before time u. Let d(1), . . . ,d(k) be the number of observed events corresponding to each time t(1), . . . ,t(k) and let r(1), . . . ,r(k) be the number of subjects at risk for the event corresponding to each time t(1), . . . ,t(k). Then the Greenwood variance formula is: var{S(u)} = {S(u)*S(u)}*{ d(1)/ [r(1)*{r(1) - d(1)}]+ . . . +d(k)/ [r(k)*{r(k) - d(k)}]}.20
Because this was a single-group pilot trial, we chose a relaxed false-positive rate of 10% for the primary end point. Furthermore, to be conservative, we were interested only in the lower bound of the CI for the true virologic success rate. For all other analyses, both the upper and lower confidence limits are of interest; thus, 2-sided CIs were used.
This study was powered so that using a 1-sided alternative and a type I error rate of 10%, a sample size of 30 patients would provide about 85% power to detect a difference between the predicted virologic success rate of atazanavir-ritonavir of 75% and a nominal success rate of 90%. We adjusted the sample size estimate, for an annual dropout rate of 8%. This rate was derived from prior ACTG studies with representative subject populations. Our accrual target was therefore 32.6 (30/0.92), or 33 patients. Power was prespecified.
The distribution of the time from simplification to any adverse events was described via the Kaplan-Meier method with pointwise 2-sided 90% CIs (using the Greenwood variance formula). The exact Wilcoxon rank-sum test21 was used to compare the number of clinic visits with detectable atazanavir concentrations and the distribution of atazanavir concentrations(>12 hours postdose) among participants who did and did not experience virologic failure. The critical P value for these analyses was <.10. Changes in CD4 cell counts and plasma lipid levels were analyzed using a 90% 2-sided CI for their respective mean values (normal theory method). Descriptive analyses were used for HIV-1 RNA in plasma and semen samples. SAS version 9, run on the SunOS 5.8 platform (SAS Institute Inc, Cary, NC), was used for all statistical analyses. P<.10 was used to determine statistical significance.
Atazanavir concentrations were used as a metric of adherence as follows: for all patients, the frequency of measurable atazanavir concentrations was determined. For example, there were 10 planned clinic visits per patient for the trial. A patient who had measurable atazanavir concentrations at each visit would have a score of 10/10 or 100%; if concentrations were only measurable for 8 of 10 visits, the score would be 8/10 or 80%. In addition, the median atazanavir concentration was determined for each patient to represent a measure of exposure across the duration of study treatment. For comparison purposes, only those concentrations obtained within the window of 12 to 24 hours after the last dose of atazanavir were used. In cases where the atazanavir concentration was below the LOQ (20 ng/mL), an arbitrary value of 5 ng/mL was assigned.
|
|
| |
| |
|
|
|