| |
Diagnostic and Clinical Implications of Anorectal Lymphogranuloma Venereum in Men Who Have Sex with Men: A Retrospective Case-Control Study
|
| |
| |
Clinical Infectious Diseases Jan 15, 2006;42:186-194
Akke K. Van der Bij,1,a Joke Spaargaren,2,a Servaas A. Morre,4 Han S. A. Fennema,3 Adrian Mindel,8 Roel A. Coutinho,1,5,7 and Henry J. C. de Vries3,6
1Department of HIV and STD Research, 2Public Health Laboratory, and 3Sexually Transmitted Disease Outpatient Clinic, Municipal Health Service of Amsterdam, 4Department of Pathology, Laboratory of Immunogenetics, Immunogenetics of Infectious Diseases Section, VU University Medical Center, Departments of 5Human Retrovirology and 6Dermatology, Academic Medical Center, University of Amsterdam, Amsterdam, and 7Center for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; and 8 Sexually Transmitted Infections Research Centre, Westmead Hospital, University of Sydney, Australia
LGV infection is a serious concern for the MSM community in Western Europe and other industrialized countries. Awareness of, screening for, and prompt treatment of LGV is crucial for the individual patient and to prevent further transmission in the wider (MSM) community.
"....HIV seropositivity, proctitis noted by proctoscopic examination, and WBC count in a Gram-stained anorectal smear specimen can be used to identify which MSM are most likely to have anorectal LGV. Systematically performed proctoscopic examination with determination of WBC count in Gram-stained anorectal smear specimens in a large group of MSM who attended a low-threshold STI clinic enabled us to perform this first systematic study on risk factors for and clinical predictors of LGV. It provides a simple strategy for LGV testing and/or (syndromic) treatment of MSM...."
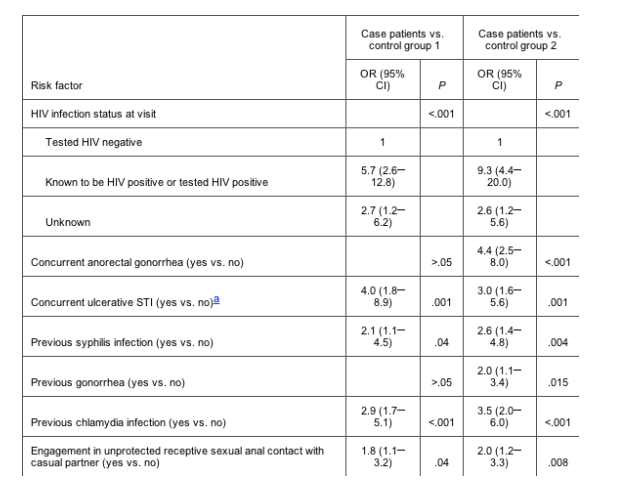
Table 2. Risk factors for anorectal lymphogranuloma venereum (LGV) in 87 men who have sex with men (MSM) who have LGV (case patients), 377 MSM who have non-LGV anorectal chlamydia (control group 1), and 2677 MSM who do not have anorectal chlamydia (control group 2), as determined by multivariate logistic regression analysis.
Lymphogranuloma venereum (LGV) is a sexually transmitted infection (STI) caused by Chlamydia trachomatis serovars L1, L2, and L3. It is endemic in parts of Africa, Asia, South America, and the Caribbean, but it has been rare in industrialized countries. However, in 2003, a cluster of cases of LGV among men who have sex with men (MSM) was reported in Rotterdam, The Netherlands [1]. Since then, there have been other reports on similar outbreaks in large cities in western Europe and the United States [27].
In contrast to urogenital chlamydia infections that are caused by C. trachomatis serovars AK and characterized by mild and often asymptomatic infection, LGV can cause severe inflammation and invasive infection, often with systemic symptoms. Depending on the site of inoculation, LGV can manifest either as an inguinal syndrome with a unilateral painful inguinal lymphadenopathy (buboes) or as an anorectal syndrome with hemorrhagic proctocolitis and hyperplasia of intestinal and perirectal lymphatic tissue. LGV responds well to extensive antibiotic treatment, but when untreated, it may cause chronic or irreversible complications, including fistulas, strictures, genital elephantiasis, frozen pelvis, or infertility [8, 9]. All of the recently reported cases of LGV infection presented as anorectal syndrome among MSM, and case reports suggest that the recent outbreak of LGV has been concentrated in sexual networks of MSM and is associated with attendance at sex parties and with HIV seropositivity [1, 3, 5]. However, predictors of LGV in the present outbreak have not been studied systematically. Therefore, we conducted a retrospective case-control study to identify risk factors for and clinical and diagnostic signs of anorectal LGV infection in MSM, to determine the implications for clinical practice.
ABSTRACT
Background. Recently, outbreaks of anorectal lymphogranuloma venereum (LGV) have occurred among men who have sex with men (MSM). This study identifies risk factors and clinical predictors of LGV to determine the implications for clinical practice.
Methods. The Chlamydia trachomatis serovars for all MSM who had anorectal chlamydia diagnosed at a sexually transmitted infection clinic in Amsterdam, The Netherlands, in 2002 and 2003 were retrospectively typed; 87 persons were infected with C. trachomatis serovar L2b and received a diagnosis of LGV. MSM infected with C. trachomatis serovars AK and who thus had non-LGV anorectal chlamydia (n = 377) and MSM who reported having receptive anorectal intercourse but who did not have anorectal chlamydia (n = 2677) served as 2 separate control groups. Risk factors and clinical predictors were analyzed by multivariate logistic regression. Receiver operating characteristic curves were used to determine clinical relevance.
Results.
HIV seropositivity was the strongest risk factor for LGV (odds ratio for patients with LGV vs. those with non-LGV chlamydia, 5.7 [95% confidence interval, 2.612.8]; odds ratio for patients with LGV vs. control subjects without chlamydia, 9.3 [95% confidence interval, 4.420.0]).
Proctoscopic findings and elevated white blood cell counts in anorectal smear specimens were the only clinically relevant predictors for LGV infection (area under the curve of the receiver operating characteristic curve, > 0.71).
Use of these 2 parameters and HIV infection status provided the highest diagnostic accuracy (for MSM with anorectal chlamydia, the area under the curve was >0.82; sensitivity and specificity were 89% and 50%, respectively).
Conclusions. LGV testing is recommended for MSM with anorectal chlamydia. If routine LGV serovar typing is unavailable, we propose administration of syndromic LGV treatment for MSM with anorectal chlamydia and either proctitis detected by proctoscopic examination, >10 white blood cells/high-power field detected on an anorectal smear specimen, or HIV seropositivity.
RESULTS
Study population. We identified 87 patients with anorectal LGV; in all cases, the C. trachomatis serovar was L2b. Three-hundred seventy-seven MSM with non-LGV anorectal chlamydia and 2677 MSM without anorectal chlamydia were included as control subjects. Compared with the 2 control groups, patients with LGV engaged more often in unprotected sex when having receptive anal intercourse with casual partners (41% vs. 20% and 17%; P < .001), were more often coinfected with HIV (60% vs. 23% and 12%; P < .001), and had more previous STIs than did control subjects (54% vs. 31% and 20%; P < .001) (table 1)
Risk factors for LGV infection. HIV seropositivity was the strongest significant independent risk factor for anorectal LGV infection among MSM, irrespective of the control group used (OR for comparison with control subjects who had non-LGV chlamydia, 5.7 [95% CI, 2.612.8]; OR for comparison with control subjects who did not have chlamydia, 9.3 [95% CI, 4.420.0]). Other independent risk factors were concurrent ulcerative disease, previously diagnosed STI, and unprotected sex when having receptive anal intercourse with casual partners (table 2).
Clinical presentation of LGV. A small number of patients with LGV presented with self-reported anorectal problems, with only 4 of these patients reporting anorectal pain or discharge. Physical examination revealed anorectal ulcers in 8 patients (9%), enlarged inguinal lymph nodes in 21 patients (24%), and proctoscopic signs of a proctitis in only 41 patients (47%). Microscopic examination of Gram-stained anorectal smear specimens revealed >10 WBC/hpf for 53 patients (61%), including 38 patients (72%) for whom >50 WBCs/hpf were present (table 1). Of the 52 HIV-positive patients, 28 (54%) reported use of antiretroviral therapy. These patients were compared with HIV-positive patients who did not receive antiretroviral therapy, and there were no significant differences in the prevalence of proctitis noted by proctoscopic examination (57% vs. 50%: P = .2) or of anorectal smears with >50 WBCs/hpf (50% vs. 52%; P = .7).
Proctoscopic signs of proctitis, enlarged inguinal lymph nodes, and the presence of >1 anorectal ulcer upon examination were significant predictors of LGV infection, irrespective of the control group used (table 3). In addition, the number of WBCs in the Gram-stained anorectal smear specimen was a significant predictor of LGV infection; when evaluating MSM with chlamydia infection, those for whom >50 WBCs/hpf were present were 5.4 times more likely to be infected with an LGV strain than with a non-LGV chlamydia strain (95% CI, 2.710.7). When patients with LGV were compared with control subjects who did not have chlamydia, those for whom >10 WBCs/hpf were present were 3.5 times more likely to be infected with LGV (95% CI, 1.86.7), and those for whom >50 WBCs/hpf were present were 13.9 times more likely to be infected with LGV (95% CI, 7.625.5). Because concurrent infections could confound the aforementioned associations, we also restricted analysis to patients without any infections other than LGV chlamydia and control subjects without any infection other than non-LGV chlamydia (table 4). This revealed similar strong associations between high WBC count, physical and proctoscopic findings, and LGV infection. Also in patients and control subjects with (co-)infections, proctoscopic findings and WBC counts were significantly associated with LGV (data not shown).
In genito-urinary medicine clinics, syndromic management of STI requires immediate blind treatment of symptomatic patients before definite test results become available. To decide which patients should start with immediate treatment for LGV, we composed ROC curves to determine the relevance of the clinical predictors in the total population of MSM who visited the STI clinic and who reported having unprotected receptive anal intercourse (n = 3141). Individually, only the number of WBCs/hpf on a Gram-stained anorectal smear specimen and proctoscopic findings were relevant predictors for LGV infection (AUC, 0.75 and 0.75, respectively). Combination of both predictors improved the clinical accuracy considerably (AUC, 0.83), as did additional combination that included the strongest independent risk factor, HIV infection status (AUC, 0.90).
In primary care settings like a general practitioners' office, a Gram-stained smear is not readily obtained, so these results are often unavailable for immediate diagnostic and therapeutic considerations. Therefore, we composed ROC curves using only those parameters ready at hand in most practices: HIV infection status, proctoscopic findings, and enlarged lymph nodes noted during physical examination. A model with proctoscopic findings and HIV infection status is as accurate in predicting anorectal LGV in an MSM population reporting receptive anal sex as a model with proctoscopic findings and the number of WBCs/hpf in an anorectal Gram-stained smear (AUC, 0.86 vs. 0.83) (figure 1). Addition of findings from an inguinal lymph node examination, however, did not further improve accuracy for LGV (AUC, 0.87).
As soon as chlamydia PCR test results become available, it is essential to differentiate between LGV and non-LGV chlamydia serovars for treatment reasons. HIV infection status, proctoscopic findings, and the number of WBCs/hpf on an anorectal Gram-stained smear specimen all had similar accuracy for predicting LGV infection among MSM with anorectal chlamydia (AUC, 0.71, 0.69, and 0.71, respectively). Use of both the number of WBCs/hpf on a Gram-stained anorectal smear specimen and proctoscopic findings improved accuracy (AUC, 0.76), and use of all 3 variables combined yielded the highest accuracy (AUC, 0.82). Application of these 3 variables in a population of MSM reporting receptive anal sex to identify the MSM who are most likely to have anorectal LGV results in an overall sensitivity of 89% and specificity of 94% (figure 2).
DISCUSSION
HIV seropositivity, proctitis noted by proctoscopic examination, and WBC count in a Gram-stained anorectal smear specimen can be used to identify which MSM are most likely to have anorectal LGV. Systematically performed proctoscopic examination with determination of WBC count in Gram-stained anorectal smear specimens in a large group of MSM who attended a low-threshold STI clinic enabled us to perform this first systematic study on risk factors for and clinical predictors of LGV. It provides a simple strategy for LGV testing and/or (syndromic) treatment of MSM. Because a substantial proportion of all STIs in The Netherlands are diagnosed at our clinic, this study is well suited to describe the spectrum of LGV in MSM [10]. Limitations of this study are that we did not know the HIV serostatus for a proportion of patients (30% of the case patients and 41% of the control subjects) and that data collection took place during a routine STI consultation. Because we did not specifically elicit data on risk behavior, symptoms, and/or medication use, underreporting of these risk factors may have occurred. This could explain, to some extent, the low proportion of complaints by patients with LGV in our study.
The first LGV cases in the Rotterdam outbreak were reported in February 2003 [1]. We describe 87 patients with LGV, the first of whom received a retrospective diagnosis in February 2002. This supports the presumption that LGV has been present for some time among MSM and may be far more common than was previously assumed [17, 18]. Diagnosis of LGV was delayed or missed because it requires tests that are not routinely performed, such as C. trachomatis serovar typing using specialized, infrequently used nucleic acid amplification tests (NAATs) or serological tests for C. trachomatis. In addition, LGV has been presumed to be an ulcerative STI presenting with inguinal lymph nodes (buboes) and systemic involvement, whereas currently, LGV in MSM mainly presented as anorectal proctitis without lymph node enlargement [17, 19].
As in previous reports [1, 3], we also found a strong association between anorectal LGV and HIV seropositivity. There are several possible explanations for this. First, sexual risk behavior has increased among HIV-positive MSM since the widespread introduction of HAART in the western world [20]. Serosorting (i.e., when HIV-positive men choose to have unprotected sex with seroconcordant partners) could have created selective "high-risk" sexual networks for STI transmission, facilitating the spread of LGV within the group of HIV-positive MSM. Second, HIV infection could operate as a biological susceptibility factor for LGV. Third, immune restoration inflammatory syndrome (i.e., clinical manifestation of a previous asymptomatic infection after the commencement of HAART [21]) may have had an effect on the sudden onset of this LGV epidemic. However, in our study, the clinical presentation of LGV was not associated with use of antiretroviral therapy in the HIV-positive LGV group. Finally, the association between LGV and HIV infection may be explained by the ulcerative character of LGV, which could facilitate transmission and acquisition of HIV [22, 23].
Because resources for LGV serovar typing are limited, LGV surveillance and treatment in Europe and other western countries is based on a probable case definition, which includes confirmed anorectal chlamydia and clinical signs resembling the anorectal or inguinal syndrome, or confirmed anorectal chlamydia and a sexual partner with confirmed LGV [24, 25]. However, our study shows that a substantial proportion of patients with LGV are asymptomatic. Signs of a clinical proctitis were visible in only 47% of the patients, and a microscopic proctitis was present in 61% of the patients. Therefore, the current case definition, which is based on clinical symptoms, may not be sufficient for surveillance and syndromic management, because asymptomatic cases will be missed. On the basis of our treatment algorithm (figure 2), syndromic management of LGV in MSM who engage in receptive anal sex should preferably be based on (1) signs of proctitis upon proctoscopic examination, and (2) one of the following findings: >10 WBCs/hpf for an anorectal Gram-stained smear specimen or HIV seropositivity. Therefore, in addition to standard STI screening and appropriate treatment procedures, we suggest immediate administration of blind antibiotic treatment for LGV (doxycycline), pending test results, for these specific groups of MSM. This approach does not imply widespread and unnecessary treatment of MSM with a non-LGV proctitis. According to the 2002 guidelines for STIs of the Centers for Disease Control and Prevention (CDC), all patients with proctitis should be prescribed doxycycline (100 mg orally twice per day for 7 days) plus ceftriaxone (125 mg given im), pending the results of laboratory tests [26]. If anorectal chlamydia is confirmed, doxycycline therapy should be continued for at least 7 days, which is adequate for non-LGV chlamydia. In addition, we recommend LGV serovar typing for all MSM with a confirmed anorectal chlamydia infection found during routine STI screening. We advise commencement or continuation of the LGV treatment regimen, pending serovar type data, for MSM with confirmed anorectal chlamydia and one of the following findings: proctoscopic proctitis, >10 WBCs/hpf in a Gram-stained anorectal smear, and HIV seropositivity. If LGV is confirmed, doxycycline therapy should be administered for a minimum of 21 days or for as long as anorectal symptoms persist. If LGV serovar testing is unavailable, blind LGV treatment is advisable in the aforementioned situations (figure 2). The strategy above also involves standard HIV testing of MSM who are at risk for LGV and other STIs. Because case reports suggest that LGV facilitates transmission of hepatitis C virus [27], hepatitis C virus infection should also be considered in all MSM with LGV.
According to our proposed algorithm, detection of C. trachomatis in rectal swabs is the first step in LGV screening, and LGV serovar typing for all MSM with confirmed anorectal chlamydia is the second. In many microbiological laboratories in continental European countries, NAAT technology is used for the detection of chlamydia in rectal swab specimens after "in house" validation [7, 13]. Recently, the Roche Amplicor has been validated for the detection of C. trachomatis in rectal specimens [11]; this will further incorporate the use of NAATs for the diagnosis of anorectal chlamydia. Subsequently, determination of the serovar for C. trachomatispositive anorectal specimens could be offered by a network of appointed laboratories that specialize in LGV. The initiation of a network of specialized laboratories for LGV diagnostics was proposed during an European Surveillance of Sexually Transmitted Infections satellite workshop on LGV research during the International Society for Sexually Transmitted Diseases Research meeting in Amsterdam on 1013 July 2005. Our recently developed LGV-specific, RT-PCRbased Taqman test could be helpful for the incorporation of LGV diagnostics into more microbiological laboratories [28]. However, compared with most European countries, in the United States, it will be more problematic to incorporate LGV diagnostics into laboratory testing, because both US Food and Drug Administration (FDA) and Clinical Laboratory Improvement Amendments (CLIA) regulations apply. The use of rectal specimens has not been evaluated for the different NAATs that have been approved by the FDA for laboratory diagnosis of C. trachomatis for use with other urogenital specimens (i.e., endocervical, male urethral, and vaginal swab specimens and urine samples obtained from men and women); thus, none of these NAATs have been approved by the FDA for use with rectal specimens (for a list of FDA-approved NAATs, see the 510K database [http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm; product code: MKZ]). Consequently, the CDC does not recommend the use of NAATs for rectal chlamydia screening until performance of the different NAATs has been evaluated with this specimen type and any specific NAAT is approved by the FDA for a rectal swab indication. However, the CDC has initiated studies to support FDA approval of NAATs for C. trachomatis detection with rectal swab specimens to alleviate the burden on individual laboratories for validating (under CLIA regulations) the NAAT that they may choose to use for testing of rectal samples.
LGV infection is a serious concern for the MSM community in Western Europe and other industrialized countries. Awareness of, screening for, and prompt treatment of LGV is crucial for the individual patient and to prevent further transmission in the wider (MSM) community.
With this in mind, future research on prevalence, natural history including complications, and straightforward diagnosis and treatment are necessary.
|
|
| |
| |
|
|
|