| |
A Once-Daily Lopinavir/Ritonavir-Based Regimen
Provides Noninferior Antiviral Activity Compared With a Twice-Daily Regimen
|
| |
| |
J Acquir Immune Defic Syndr Aug 31 2006
Margaret A. Johnson, MD,* Joseph C. Gathe, Jr., MD, Daniel Podzamczer, MD,
Jean-Michel Molina, MD, PhD, Christian T. Naylor, BA,k Yi-Lin Chiu, PhD,k
Martin S. King, PhD,k Thomas J. Podsadecki, MD,k George J. Hanna, MD,k
and Scott C. Brun, MDk
From the *Royal Free Center for HIV Medicine, London, United Kingdom;
Therapeutic Concepts, P.A., Houston, TX; Hospital Universitari de
Bellvitge, L'Hospitalet, Barcelona, Spain; Hoo pital Saint Louis, Paris,
France; and kAbbott Laboratories, Abbott Park, IL.
Subjects were centrally randomized at a 3:2 ratio to receive open-label lopinavir/ritonavir soft-gelatin capsules at a dose of 800/200 mg administered once daily (n = 120) or 400/100 mg administered twice daily (n = 80).
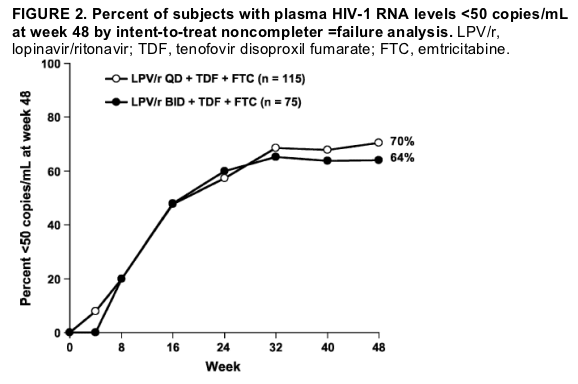
Antiviral Activity
In the primary analysis at week 48, 81 (70%) of 115 subjects in the once-daily group and 48 (64%) of 75 of subjects in the twice-daily group demonstrated plasma HIV-1 RNA levels less than 50 copies/mL (P = 0.43; Fig. 2). The 95%
confidence interval for the difference (once daily 2 twice daily) in response was 27% to 20%. Results were similar for the secondary end point based on the FDATLOVR algorithm, because 82 (71%) of 115 subjects in the once-daily group and 49 (65%) of 75 subjects in the twice-daily group achieved HIV-1 RNA levels less than 50 copies/mL through week 48 (P = 0.42; 95% confidence interval: 28% to 20%).
Based on logistic regression analysis, there was no statistically significant association between virologic response and lopinavir median Ctrough. Response was not dependent on demographic characteristics (gender, race/ethnicity, and age) and baseline disease characteristics (HIV-1 RNA level, CD4 cell count, and baseline hepatitis infection status) for all subjects combined or separately within each treatment group (data not shown).
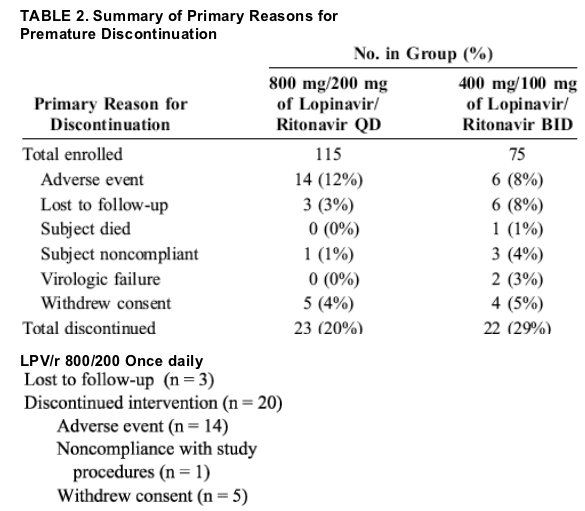

Of 244 subjects screened, 196 met eligibility criteria and were randomized between August 7, 2002 and December 3, 2002 (Fig. 1). Three subjects assigned to each regimen never received study drug; the remaining 190 subjects (115 in the once-daily group and 75 in the twice-daily group) were included
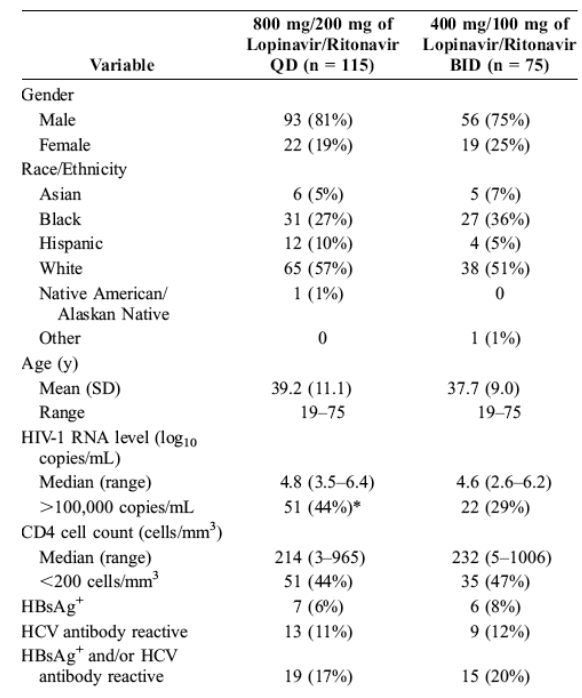
in the analysis. One subject in the once-daily group selfswitched from once-daily to twice-daily dosing of lopinavir/ritonavir from week 32 to week 48. Baseline characteristics were similar in the 2 treatment groups (Table 1). A total of 23 subjects (20%) in the once-daily group and 22 subjects (29%) in the twice-daily group discontinued the study through week 48 (Table 2). More subjects in the twice-daily group (8%) were lost to follow-up compared with the once-daily group (3%), and discontinuation attributable to adverse events was more common in the once-daily group (12%) compared with the twice-daily group (8%), although neither difference was statistically significant. Adverse events resulting in discontinuation were generally gastrointestinal in nature.
ABSTRACT
Objective: To evaluate the safety and noninferiority and to explore the efficacy of administration of once-daily versus twice-daily lopinavir/ ritonavir (LPV/r) in antiretroviral-naive HIV-1-infected subjects.
Design: Randomized, open-label, multicenter, comparative study.
Methods: One hundred ninety antiretroviral-naive subjects with plasma HIV-1 RNA level .1000 copies/mL and any CD4+ cell count were randomized to lopinavir/ritonavir at a dose of 800/200 mg administered once daily (n = 115) or lopinavir/ritonavir at a dose of 400/100 mg administered twice daily (n = 75). Subjects also received tenofovir disoproxil fumarate (TDF) at a dose of 300 mg and emtricitabine (FTC) at a dose of 200 mg administered once daily.
Results: The median baseline plasma HIV-1 RNA level and CD4+ count were 4.8 log10 copies/mL and 216 cells/mm3, respectively. Before week 48, 20% (once daily) and 29% (twice daily) subjects discontinued. Virologic responses of the subjects through 48 weeks were comparable; 70% (once daily) and 64% (twice daily) achieved an HIV-1 RNA level <50 copies/mL by intent-to-treat, noncompleter =failure analysis. No subject demonstrated LPVor TDF resistance but 3 subjects (2 in the once-daily group, 1 in the twice-daily group) demonstrated FTC resistance. Mean increases in CD4 count were similar. Diarrhea (16% in the once-daily group, 5% in the twice-daily group; P = 0.036) was the most common moderate or severe study drug-related adverse event.
Conclusions: Through 48 weeks, a once-daily regimen of lopinavir/ritonavir + TDF + FTC appears to have similar virologic and immunologic responses in antiretroviral-naive subjects as the same regimen with lopinavir/ritonavir administered twice daily. Both regimens were relatively well tolerated, and no LPVor TDF resistance was observed.
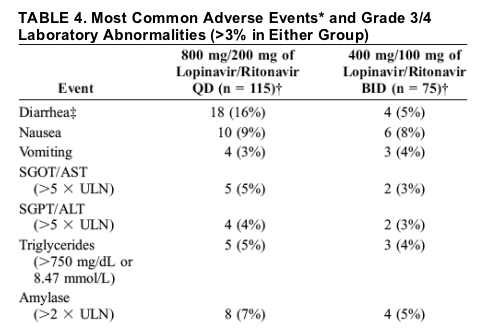
Adverse Events
The most common adverse events of at least moderate severity and with a possible, probable, or unknown relation to study drug were gastrointestinal in nature (Table 4). Nine subjects in the once-daily group (8%) and 2 in the twice-daily group (3%) discontinued the study because of gastrointestinal adverse events. Diarrhea of moderate or greater severity occurred more frequently in the once-daily group (16%) compared with the twice-daily group (5%; P = 0.036).
Diarrhea generally occurred early and was self-limiting; for most of the subjects with diarrhea (12 of 22 subjects), initial onset was during the first week, and only 3 subjects had new onset events after week 16. From weeks 16 to 48, the prevalence of diarrhea remained at 6% or less in the once-daily group and at 2% or less in the twice-daily group, which includes subjects with diarrhea ongoing at the time of discontinuation.
There was 1 death in the study. A subject in the twicedaily group died of multiorgan failure after 6 weeks on study, after a diagnosis of adenocarcinoma. The subject was receiving chronic prednisone therapy for myositis. The event was considered unrelated to study drugs.
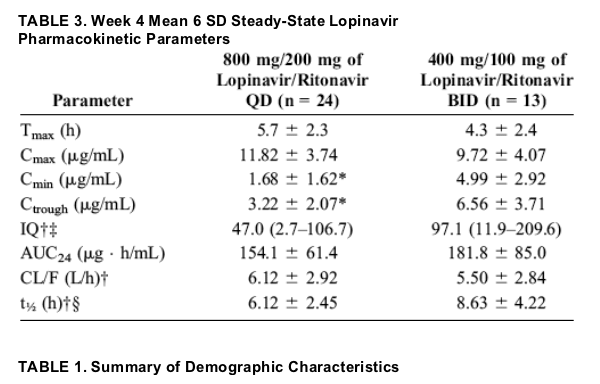
Pharmacokinetics
Results of week 4 steady-state lopinavir pharmacokinetic parameters for 37 subjects are presented (Table 3). Among the parameters tested, Ctrough and Cmin were statistically significantly lower during once-daily dosing compared with
twice-daily dosing (P <0.003). Neither lopinavir AUC24 nor Cmax was statistically significantly different between the regimens (P = 0.113 for Cmax and 0.176 for AUC24). The overall median IQ was significantly lower for the once-daily group (48.1) compared with the twice-daily group (86.5; P <0.001), corresponding to a Ctrough of 4.37 and 6.64 mg/mL, respectively.

Laboratory Abnormalities
Statistically significant increases in total cholesterol were composed of significant increases in high-density lipoprotein (HDL) cholesterol (0.1 mmol/L [3 mg/dL] in the once-daily group and 0.2 mmol/L [6 mg/dL] in the twice-daily group) and low-density lipoprotein (LDL) cholesterol (0.4 mmol/L [14 mg/dL] in each group). Lipid increases were observed early, in the first 8 weeks, with little change in mean values subsequent to that time. Week 48 median values for total cholesterol and LDL cholesterol were 4.6 and 2.7 mmol/L, respectively (179 and 106 mg/dL, respectively), for the once-daily group and 4.8 and 3.0 mmol/L, respectively
(187 and 117 mg/dL, respectively), for the twice-daily group. Week 48 median triglyceride values were 1.9 mmol/L (172 mg/dL) in the once-daily group and 2.0 mmol/L (176 mg/dL) in the twice-daily group. Three subjects were using lipidlowering agents at baseline, and 1 additional subject in each treatment group initiated lipid-lowering agents during the first 48 weeks of the study.
In an analysis of combined treatment groups, 86% of subjects had LDL cholesterol levels in the optimal or near-optimal range (,130 mg/dL or 3.37 mmol/L)13 at baseline, compared with 74% at week 48. For HDL cholesterol, the proportion of subjects with low values (<40 mg/dL or 1.04 mmol/L) decreased from 58% at baseline to 42% at week 48, whereas the proportion of subjects with high values (>/=60 mg/dL or 1.55 mmol/L) increased from 8% to 13% over the same period. Grade 3 or higher elevations of total cholesterol (>300 mg/dL or 7.77 mmol/L) and triglycerides (>750 mg/dL or 8.47 mmol/L) were uncommon and occurred in less than 3% and 5% of subjects in either group, respectively (see Table 4). At week 48, 87% of subjects maintained grade 0 to 1 or lower values for total cholesterol (<240 mg/dL or 6.22 mmol/L) and triglycerides (<400 mg/dL or 4.52 mmol/L).
Small but statistically significant increases in mean serum creatinine were observed from baseline (0.86 mg/dL) to week 48 (0.91 mg/dL; P <0.001), with no statistically significant difference between groups. Decreases in creatinine
clearance, as estimated by the Cockcroft-Gault equation (120 mL/min at baseline, 112 mL/min at week 48; P , 0.001), and glomerular filtration rate, as estimated by the modification on diet in renal disease (MDRD) equation (112 mL/min/1.73 m2 at baseline, 104 mL/min/1.73 m2 at week 48; P , 0.001), were
also observed with no statistically significant differences between the once-daily and twice-daily groups.
Overall, for 98% of subjects, the maximum creatinine value was 3.0 mg/dL and acute renal failure (ARF). ARF occurred at week 38 in a 54-year-old male subject with a history of diabetes mellitus and a baseline creatinine clearance of 114 mL/min, who required temporary hemodialysis. Renal biopsy demonstrated interstitial nephritis. The subject discontinued the study, and creatinine levels returned to near normal. ARF also occurred at week 34 in a 75- year-old male subject with a history of diabetes mellitus and a baseline creatinine clearance of 40 mL/min, who was begun on full-dose TDF. The subject received 1 hemodialysis session. Renal biopsy demonstrated signs compatible with diabetic nephropathy. Lopinavir/ritonavir was restarted, but TDF was replaced by stavudine at a dose of 30 mg administered twice daily and the dose of FTC was reduced to 200 mg every 72 hours. His creatinine level continued to decrease to near-normal levels. TDF dosing recommendations implemented after initiation of this study indicate that administration of TDF every other day would have been most appropriate for this subject based on creatinine clearance.
Viral Drug Resistance
Twenty-two subjects (11 in the once-daily group, 11 in the twice-daily group) met criteria for genotypic resistance testing. For one subject in the twice-daily group, no specimen was available for testing; of note, the subject continued on the
same regimen, and the plasma HIV-1 RNA level was subsequently less than 50 copies/mL at week 48. In 6 subjects (3 in the once-daily group, 3 in the twice-daily group), resistance testing failed because of low HIV-1 RNA levels (median = 625 copies/mL, range: 533-1029 copies/mL). Results were therefore available for 15 subjects, representing 8 subjects in the once-daily group and 7 in the twice-daily group. No subject in either group demonstrated lopinavir or TDF resistance. Development of FTC resistance was observed in 2 subjects in the
once-daily group and in 1 subject in the twice-daily group.
DISCUSSION
Prior studies have demonstrated that lopinavir/ritonavir at a dose of 400/100 mg administered twice daily in combination with 2 nucleoside reverse transcriptase inhibitors (NRTIs) provides a potent, well-tolerated, and durable treatment regimen for antiretroviral naive patients.2,3 In this clinical trial conducted in previously untreated HIV-1-infected individuals, an entirely once-daily regimen consisting of the lopinavir/ritonavir soft-gelatin capsule at a dose of 800/200 mg with TDF and FTC was noninferior to lopinavir/ritonavir at a dose of 400/100 mg administered twice daily with the same NRTI backbone. In the primary analysis at week 48, 70% of subjects in the once-daily group and 64% of subjects in the twice-daily group demonstrated HIV-1 RNA levels less than 50 copies/mL. The lower bound of the 95% confidence interval for the difference (once daily 2 twice daily) falls at 27%, which is well within the 210% to 212% boundary commonly accepted as establishing noninferiority for antiretroviral treatment regimens administered to subjects new to therapy.10 Immunologic recovery, as measured by CD4 cell increases from baseline, was similar in the 2 treatment arms.
These regimens using the soft-gelatin capsule formulation of lopinavir/ritonavir were safe and relatively well tolerated. Overall discontinuations were similar between groups, although a greater number of discontinuations for adverse
events (primarily gastrointestinal) and a significantly higher rate of diarrhea were observed in the once-daily group. No significant difference in lipid profiles was observed between the once-daily and twice-daily groups. LDL cholesterol effects were modest in both groups, with findings suggesting that only an additional 12% of the study population shifted out of the optimal LDL cholesterol range (<130 mg/dL or 3.37 mmol/L)13 during 48 weeks of therapy. Additionally, HDL cholesterol increases were observed in both groups. The most pronounced lipid effect was observed with respect to triglycerides. Almost 90% of subjects maintained triglyceride values <400 mg/dL (4.52 mmol/L), however. In this study, the lipid increases were less than have been observed when lopinavir/ritonavir is administered with thymidine analogue nucleosides, such as stavudine.3 The use of lipid-lowering agents was uncommon in both study arms, and no subject discontinued the study because of lipid elevations.
The distribution of creatinine values observed was comparable to that reported in earlier trials with TDF.14 Two subjects developed ARF during the first 48 weeks of the study. The clinical presentation and pathophysiologic mechanism leading to renal failure seem to differ in these 2 subjects. Therefore, these cases did not seem to represent an aggregate signal for synergistic renal toxicity attributable to coadministration of lopinavir/ritonavir and TDF.
Despite differences in the NRTI backbone, pharmacokinetic results observed in this study (using once-daily TDF and FTC) were consistent with observations made in a prior pilot study evaluating once-daily lopinavir/ritonavir (and using
an NRTI backbone of twice-daily stavudine and lamivudine) in HIV-1-infected subjects.8 Thus, there is no evidence to suggest that these different NRTI backbones affect lopinavir plasma concentrations differently. Additionally, although the Ctrough achieved with lopinavir with once-daily dosing is significantly lower than that achieved with twice-daily dosing, a pharmacokinetic/pharmacodynamic analysis did not suggest an association of a lower lopinavir Ctrough with reduced virologic response in this study. This observation suggests that for antiretroviral-naive patients, the Ctrough of lopinavir achieved with the once-daily dosing regimen does not reside
on the steep portion of the concentration-response curve. The lack of development of lopinavir resistance in either dosing group and the low rate of FTC resistance through 48 weeks of therapy further support this theory and suggest that the subsequent use of protease inhibitor-based treatment regimens
would not be limited through the use of either lopinavir/ritonavir dosing schedule as initial therapy for HIV-1. The absence of resistance in this study is also
consistent with reports that boosted protease inhibitor-based regimens are associated with lower rates of resistance than nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens.15
Certain limitations of this study should be considered. The sample size provided only 60% power to determine noninferiority of the once-daily group in the primary efficacy analysis, typical of a phase 2b study but lower than the 80% to 90% power used in phase 3 studies. Power calculations are of less interest once a trial is complete, however; in particular, the study results demonstrated noninferiority of the 2 regimens despite the prestudy power calculations.16 With any noninferiority study, the performance of the control arm may also limit interpretation. Notably, the lopinavir/ritonavir-based twice-daily reference arm in this study performed similar to a lopinavir/ritonavir-based twice-daily regimen evaluated in a large, double-blind, randomized, controlled phase 3 study
(67% <50 copies/mL at week 48),3 indicating that the twice-daily dose arm in this study provided a valid control group. Although no difference was seen in efficacy between the once-daily and twice-daily groups through 48 weeks, the analysis
does not address the impact that lower Ctrough and IQ might have on longer term follow-up.
With the increasing number of available once-daily NRTIs, interest in once-daily protease inhibitor-based regimens is likely to grow. More convenient treatment regimens may lead to improved patient adherence, and thus to improved long-term efficacy. Results of the current study suggest that once-daily dosing of lopinavir/ritonavir is a suitable option for antiretroviral-naive patients initiating HIV-1 therapy. The new lopinavir/ritonavir tablet formulation (reduced pill count from 6 capsules to 4 tablets per day, reduced pharmacokinetic variability, no need for refrigerated storage, and dosed without regard to meals) may have fewer gastrointestinal adverse effects17 and may further increase the convenience of a lopinavir-based once-daily regimen for patients.
|
|
| |
| |
|
|
|