| |
Diet & Exercise Counseling Program Improved Cardiovascular Risk Indices and Lipodystrophy
|
| |
| |
Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome
AIDS: Volume 20(14) 11 September 2006 p 1843-1850
Fitch, Kathleen Va; Anderson, Ellen Jb; Hubbard, Jane Lb; Carpenter, Sara Ja; Waddell, William Rc; Caliendo, Angela Md; Grinspoon, Steven Ka
From the aProgram in Nutritional Metabolism, USA
bGeneral Clinical Research Center, USA
cPhysical Therapy Department, Massachusetts General Hospital, Boston, MA, USA
dEmory University School of Medicine, Atlanta, GA, USA.
".....Waist circumference is known to be a significant cardiac risk factor in non-HIV-infected patients...central adiposity is associated with significant metabolic abnormalities...Physical activity is important to prevent chronic diseases including CVD and diabetes, and low levels of physical activity may be considered a risk factor for developing these diseases....
....this study found lifestyle modification sufficient to improve caloric intake, saturated fats and fiber intake and to increase physical activity, improves waist circumference, blood pressure and HbA1C among HIV-infected patients with the metabolic syndrome....lifestyle modification was associated with a significant decrease in HbA1C, which may further reduce cardiovascular and diabetes risk in this population....Earlier data among non-HIV-infected patients demonstrated that engagement in moderate to brisk exercise for at least 30 min a day, and consumption of a diet high in cereal fiber, omega-3 fatty acids, and a high ratio of polyunsaturated fatty acids to saturated fat was associated with a low incidence of myocardial infarctions...previous research: The DPP demonstrated that lifestyle modification, with a goal of 7% weight loss and at least 150 min of activity a week reduced the incidence of diabetes mellitus by 58% among patients with impaired glucose tolerance...and...the program of dietary and exercise intervention, including the restriction of fat to 26%, saturated fat to less than 6%, and the performance of physical activity equal to five sessions of 20 min/week at 80-90% maximum predicted heart rate, increased insulin sensitivity...Among HIV-infected patients, a recent report demonstrated a decrease in total abdominal fat, waist-to-hip ratio, body mass index, total cholesterol, and increased fitness and muscle strength with progressive cardiovascular and resistance training for one hour and 15 min three times a week, and a diet with total fat intake less than or equal to 30% of total calories, a saturated fat intake less than or equal to 10% of total calories, and a fiber intake greater than 25 g per day...."
Abstract
Objectives: A large percentage of HIV-infected patients receiving HAART develop the metabolic syndrome. In this study, we sought to determine whether lifestyle modification improves metabolic syndrome criteria, including waist circumference, blood pressure, fasting blood sugar, triglycerides, and HDL-cholesterol among HIV-infected patients with the metabolic syndrome.
Design: We conducted a randomized, 6-month study in HIV-infected patients with metabolic syndrome as defined by the National Cholesterol Education Program. Subjects were randomly assigned to an intensive lifestyle modification program, which included weekly one-on-one counseling sessions with a registered dietician, or observation (control group).
Methods: Metabolic syndrome criteria and cardiovascular parameters, including blood pressure, body composition, submaximal stress testing, lipids and other biochemical parameters were determined.
Results:
Thirty-four patients were randomly assigned and 28 subjects completed the study.
Compared with the control group, subjects randomly assigned to the lifestyle modification program demonstrated significant decreases in:
- waist circumference (-2.6 ± 1.1 versus 1.2 ± 1.0 cm, P = 0.022),
- systolic blood pressure (-13 ± 4 versus 4 ± 4 mmHg, P = 0.008),
- hemaglobin A1C (-0.1 ± 0.1 versus 0.2 ± 0.1%, P = 0.017),
- lipodystrophy score (-1.2 ± 0.3 versus 0.9 ± 0.6, P = 0.006) and
- increased activity (17.7 ± 14.3 versus -33.1 ± 12.7 metabolic equivalents, P = 0.014) as measured by the Modifiable Activity Questionnaire,
- but lipid levels did not improve.
Conclusion:
These data demonstrate that intensive lifestyle modification significantly improved important cardiovascular risk indices in HIV-infected patients with the metabolic syndrome. Lifestyle modification may be a useful strategy to decrease cardiovascular risk in this population.
Introduction
The metabolic syndrome refers to a constellation of cardiovascular disease (CVD) risk factors, including increased abdominal girth, disorders of glucose and lipid metabolism, and hypertension. Each of these risk factors individually has been shown to increase cardiovascular risk [1,2]. In accordance with this, a recent report has shown that waist circumferences were closely associated with the risk of myocardial infarction even after adjustment for other risk factors [3]. In addition, the metabolic syndrome itself has been shown to be a powerful independent risk factor for cardiovascular morbidity and mortality [4]. Approximately 25% of the US population currently has the metabolic syndrome as defined using the National Cholesterol Education Program (NCEP) definition.
Combination antiretroviral therapy (ART) has changed the course of HIV infection, and has led to a significant reduction in morbidity and mortality. However, the use of ART is known to be associated with changes in fat distribution and metabolic abnormalities, including insulin resistance, dyslipidemia, and increased blood pressure [5-7]. Therefore, HIV-infected patients share several characteristics of the metabolic syndrome including increased waist circumference, insulin resistance, dyslipidemia, and hypertension. The prevalence of the metabolic syndrome in HIV-infected patients has been reported to range from 17 to 45.5% [8-10].
Despite clear data and recommendations for non-HIV-infected patients with regard to the management of metabolic abnormalities, little information exists on the utility of diet and exercise as a treatment for the metabolic abnormalities in HIV-infected patients. To our knowledge, no previous study of lifestyle modification has been performed among HIV-infected patients meeting the NCEP definition of the metabolic syndrome. We therefore sought to evaluate the effect of a 6-month comprehensive lifestyle modification program modeled after the Diabetes Prevention Program (DPP) in HIV-infected patients with the metabolic syndrome [11]. The program was designed to improve metabolic parameters through dietary modification and increased physical activity.
Subjects
Ninety-two HIV-infected men and women were screened to determine eligibility for a 6-month randomized trial to evaluate the effects of a lifestyle modification program between August 2003 and December 2005. Eligibility requirements included: previously documented HIV infection, age between 18 and 65 years, receiving stable combination ART for more than one month, and the demonstration of NCEP Adult Treatment Panel III metabolic syndrome, as defined by three out of five of the following: (i) waist circumference greater than 102 cm in men and 88 cm in women; (ii) triglyceride levels of 150 mg/dl or greater or current lipid-lowering drug treatment; (iii) HDL-cholesterol less than 40 mg/dl in men and 50 mg/dl in women; (iv) blood pressure 130/85 mmHg or greater, or current antihypertensive drug treatment; and (v) fasting glucose level of 110 mg/dl or greater.
Subjects were excluded if they had had any new serious opportunistic infection within the past 6 weeks, a history of unstable angina, aortic stenosis, uncontrolled hypertension or other contraindication to increased physical activity, current therapy with insulin, other diabetic agent, or fasting blood sugar greater than 126 mg/dl, alanine aminotransferase levels more than five times the upper limit of normal, creatinine greater than 2.0 mg/dl, required parenteral nutrition or glucocorticoid therapy, estrogen, progestational derivative, or ketoconazole use within 3 months of the study, current substance abuse, hemoglobin less than 10 mg/dl, were pregnant or actively seeking pregnancy, breastfeeding, or using supraphysiological doses of testosterone or physiological testosterone replacement for less than 3 months.
A two-step screening process was developed to identify eligible participants. Subjects were recruited through HIV clinics and providers in the Boston area, HIV community service programs, as well as advertisements in the greater Boston region. Subjects responding to community announcements or referred by HIV providers underwent a phone screen to determine potential eligibility. Appropriate candidates for the study were then scheduled for a formal outpatient screening visit to determine eligibility. Informed written consent was obtained before study participation, and the protocol was approved by the Massachusetts General Hospital Human Research Committee and the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects.
Of the 92 subjects screened, 50 were ineligible, eight declined to participate, six withdrew and 28 completed the protocol (Fig. 1). Forty-one of the ineligible subjects did not meet the definition of the metabolic syndrome. Other reasons for ineligibility included alanine aminotransferase levels five times the normal (N = 2), fasting blood glucose greater than 126 mg/dl (N = 1), lost to follow-up (N = 5) or other (N = 1).
Design
All study participants completed a detailed metabolic assessment before random selection. After baseline assessment, subjects were randomly assigned to lifestyle modification or control (no lifestyle modification) group stratified by fasting blood sugar (≦ 110 or ≥ 110 mg/dl) and sex. Testing included the determination of fasting lipids, glucose, hemoglobin A1C (HbA1C), hemoglobin, alanine aminotransferase, creatinine, as well as the CD4 cell count and HIV viral load. Insulin levels were also measured and a Homeostasis Model Assessment of insulin resistance was calculated. Weight, height, iliac waist and hip circumference, and resting blood pressure were measured. Lipodystrophy scores were calculated based on an evaluation of face, neck/shoulders, arms, abdomen, and hips/legs, with graded values between 0 and 2 for fat loss or accumulation [12]. Identical assessments were performed at the 6-month visit.
In addition to the baseline and 6-month assessments, subjects completed a brief safety visit 3 months after randomization. Subjects were evaluated for any potential adverse effects of the lifestyle modification program. An interval medical history was obtained and blood samples were obtained for a complete blood count, creatinine, and alanine aminotransferase.
Study intervention
Eligible participants were randomly assigned to one of two groups, lifestyle modification or control.
The goals for the participants assigned to the lifestyle modification group were derived from a combination of recommendations from the American Association of Clinical Endocrinologists (AACE), NCEP Adult Treatment Panel III guidelines, and the DPP study as follows: healthy eating, less than or equal to 35% of total calories from fat, less than 7% of total calories from saturated fat, up to 10% of total calories from polyunsaturated fat, with emphasis on sources of omega 3 fatty acids and a reduction in trans fatty acid intake, up to 20% of total calories from monounsaturated fat, 25-35 g of soluble and insoluble fiber per day, 3 h of physical activity per week at moderate intensity, greater than or equal to 10,000 steps in daily activity, measured by pedometer, and self monitoring.
Those randomly assigned to lifestyle modification attended weekly one-on-one counseling sessions for 6 months, covering a core curriculum modeled after the DPP study 16-week core curriculum intervention [11]. The DPP core curriculum was modified to focus on healthy eating with a limited emphasis on weight loss. The initial core curriculum sessions were completed within the first 18 weeks (4.5 months), with weekly sessions by the dietician staff at the Massachusetts General Hospital General Clinical Research Center (MGH GCRC). Each lesson was recapped in the first 12-15 min of the session immediately after, and the entire core curriculum was repeated for reinforcement between weeks 19 and 24 of the study (Table 1). Registered dieticians were trained in research methodologies, including motivational interviewing and behavioral modification. The control group received a one time counseling session with the dietician staff at the baseline visit and monthly phone calls from the study investigator for follow-up throughout the 6 months of the study. Subjects were permitted to continue baseline levels of regular exercise during the protocol.
Dietary and exercise data
Self-reported levels of physical activity were assessed at baseline and 6 months using the Modifiable Activity Questionnaire (MAQ) [13]. Physical activity level was calculated as the product of the duration and frequency of each activity (in hours per week), weighted by an estimate of the metabolic equivalent (MET) of that activity and summed for all activities performed, with the result expressed as the average MET-hours per week.
A submaximal exercise stress test was conducted on a cycle ergometer to measure endurance [14,15]. Subjects cycled at 50 revolutions per minute and the workload was progressively increased in increments of 50 Watts, starting at 50 Watts in stages lasting 3 min. At the end of each stage, separate readings of heart rate and blood pressure were measured, and rate of perceived exertion was measured using the BORG Rate of Perceived Exertion Scale. Once subjects became fatigued or reached their submaximal heart rate (220 - age X 0.85), the test was stopped and separate readings of heart rate and blood pressure were measured at 1, 3, and 5 min of recovery. Throughout the test, a 3-lead electrocardiogram was monitored for safety purposes. Exercise time was calculated based on duration (minutes) each subject rode during the submaximal bicycle test. Weight-adjusted maximum oxygen consumption (VO2max; ml/kg per minute) for the measurement of cardiorespiratory fitness was calculated using the following formula: VO2max ml/kg per minute = [((Watts in kg/m·per minute) X 2.0 ml/min) + (3.5 ml/kg per minute X mass in kg)] per kg based on the American College of Sports Medicine Equation for the estimation of VO2max during cycle ergometry [14,16]. Submaximal exercise testing was performed at baseline and 6 months for all enrolled subjects.
Dietary intake information was assessed by a 7-day self-documented food record, and reviewed by a registered dietician, and then analysed for protein, carbohydrate, fiber, fat and micronutrient intake using a computerized dietary database. Dietary intake data were collected using Nutrition Data System for Research (NDS-R) software versions 5.0_35 and 2005, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota, USA. Final calculations were completed using the NDS-R version 2005. The NDS-R time-related database updates analytic data while maintaining nutrient profiles true to the version used for data collection. Food records were completed weekly for those in the lifestyle modification group and every 3 months for those in the control group.
Laboratory methods
Total cholesterol was measured by enzymatic hydrolysis (Dade Dimension, Wilmington, Delaware, USA); serum triglycerides were measured using a lipase enzymatic method (Dade Dimension); HDL-cholesterol was measured after the precipitation of LDL-cholesterol, and LDL-cholesterol was calculated indirectly. Glucose was measured with a hexokinase reagent kit (Dade Dimension). HbA1C was measured by chromatotographic separation of HbA1C on a cation exchange cartridge (Bio-Rad Laboratories, Hercules, California, USA). Insulin was measured by radioimmunoassay (Diagnostic Products Corp., Los Angeles, California, USA). The CD4 cell count was determined by flow cytometry (Becton Dickinson Biosciences, San Jose, California, USA) and viral load was measured by the Amplicor HIV-1 Monitor v1.5 test (Roche Molecular Systems Inc., Indianapolis, Indiana, USA).
Anthropometric determinations were made in triplicate using an inelastic tape. A wall-mounted stadiometer and digital scale were also used. All measurements were performed by the dietician staff of the MGH GCRC.
Biostatistical analysis
A comparison of demographic variables was made at baseline, using Student's t-test for continuous variables and the chi-square test for non-continuous variables. Changes in treatment effects were compared between lifestyle and control groups using Student's t-test. For variables with levels that differed between the groups at baseline, results were confirmed using analysis of covariance (ANCOVA), controlling for baseline values. The primary endpoint was a change in waist circumference. With 28 evaluable subjects, the study was powered at 85% to detect a 0.1 cm change in waist circumference, at P = 0.05 significance level, based on previous studies assessing a change in waist circumference in a similar population of HIV-infected patients [17]. Outlier analysis was performed using the Dixon criterion [18].
Results
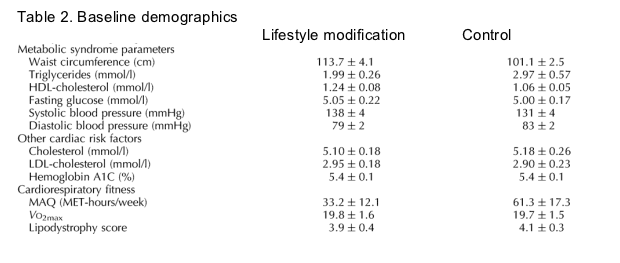
Subjects were matched at baseline for age, duration of HIV infection, and CD4 cell count (Table 2). Seventy-two per cent of the control group and 75% of the lifestyle modification group reported a history of hypertension. Thirty-three per cent of the control group and 44% of the lifestyle modification group reported a history of hyperlipidemia. The differences between groups at baseline for a history of hypertension and hyperlipidemia were not statistically significant (P > 0.05). In the control group, 39% were current smokers, whereas 50% were current smokers in the lifestyle modification group. Seventy-two per cent of the control group reported current participation in regular exercise whereas 63% of the lifestyle modification group reported regular exercise. The subjects were representative of HIV-infected patients seen in Massachusetts, in terms of age, race, and treatment status. The subjects were not enrolled in other studies. 69% in the Lifestyle modification group were African-American and 56% were African-American in the Control group. 19% in the Lifetsyle modification group and 44% in the Control group were Caucasian. CD4 count was 550-625 and HIV RNA was 2.8 to 3.0 log. 50-56% were taking anti-hypertensive agents, and 6% in the Lifestyle modification group and 11% in the Control group were taking lipid-lowering drugs.
Among subjects completing the protocol, the mean percentage compliance with counseling sessions was 75%. Subjects in the lifestyle group demonstrated a reduction in caloric intake (-347 ± 171 kcal/day, P = 0.068), percentage of calories from saturated fat (-2 ± 1%, P = 0.040) and increase in total fiber intake (4 ± 2 g/day, P = 0.057). In contrast, no significant changes were seen in these parameters among subjects in the control group (23 ± 125 kcal/day, P = 0.854 for the change in total caloric intake; 0 ± 1%, P = 0.941 for the change in the percentage of calories from saturated fat; 0 ± 2 g/day, P = 0.847 for the change in total fiber intake). Four subjects in the lifestyle modification group versus two subjects in the control group withdrew from the study. In the lifestyle modification group, two subjects were lost to follow-up, one experienced a death in the family and another became pregnant during the study. In the control group, one subject was lost to follow-up and one subject was withdrawn because of anemia associated with combination interferon/ribavirin treatment. No other patients were receiving interferon therapy.
Compared with the control group, subjects randomly assigned to the lifestyle modification group demonstrated a significant reduction in systolic blood pressure(-13 ± 4 versus 4 ± 4 mmHg, P = 0.008; Fig. 2), waist circumference (-2.6 ± 1.1 versus 1.2 ± 1.0 cm, P = 0.022), HbA1C (-0.1 ± 0.1 versus 0.2 ± 0.1%, P = 0.017), improved activity as measured by the MAQ (17.7 ± 14.3 versus -33.1 ± 12.7 MET, P = 0.014), and lipodystrophy score (-1.2 ± 0.3 versus 0.9 ± 0.6, P = 0.006; Table 3). Although waist circumference was different in the two groups at baseline, the significant change between groups was confirmed by ANCOVA controlling for baseline waist circumference. Cardiorespiratory fitness measured by VO2max tended to increase more in the lifestyle modification group than the control group. Lipid levels and fasting glucose did not change significantly in the lifestyle group compared with the control group (Table 3). Insulin levels (-4.4 ± 4.9 versus 0.2 ± 1.5 μIU/ml) and Homeostasis Model Assessment of insulin resistance (-0.9 ± 1.2 versus 0.1 ± 0.3, lifestyle modification versus control, respectively) improved in the subjects randomly assigned to the lifestyle program compared with the control group, but these changes were not statistically significant (P > 0.05). No changes in safety or immune function parameters were observed. The study was well tolerated and no adverse events related to the study occurred.
Discussion
The modification of CVD risk factors has become an important aspect of HIV management as more studies establish that there is an increased CVD risk among HIV-infected patients [5,10,19]. In this study, we demonstrated that lifestyle modification sufficient to improve caloric intake, saturated fats and fiber intake and to increase physical activity, improves waist circumference, blood pressure and HbA1C among HIV-infected patients with the metabolic syndrome.
Earlier data among non-HIV-infected patients demonstrated that engagement in moderate to brisk exercise for at least 30 min a day, and consumption of a diet high in cereal fiber, omega-3 fatty acids, and a high ratio of polyunsaturated fatty acids to saturated fat was associated with a low incidence of myocardial infarctions [20]. Assessment of lifestyle prevention as a primary prevention for coronary heart disease demonstrated that 82% of all coronary events were associated with the lack of adherence to a low-risk lifestyle pattern [20]. The DPP demonstrated that lifestyle modification, with a goal of 7% weight loss and at least 150 min of activity a week reduced the incidence of diabetes mellitus by 58% among patients with impaired glucose tolerance [21]. In the Finnish Diabetes Prevention study [22], in which individualized counseling aimed at reducing weight, the total intake of fat, and saturated fat, and increasing the intake of fiber and physical activity, the risk of diabetes was decreased 58% among overweight patients with impaired glucose tolerance. McAuley et al. [23] demonstrated that a program of dietary and exercise intervention, including the restriction of fat to 26%, saturated fat to less than 6%, and the performance of physical activity equal to five sessions of 20 min/week at 80-90% maximum predicted heart rate, increased insulin sensitivity.
Among HIV-infected patients, a recent report demonstrated a decrease in total abdominal fat, waist-to-hip ratio, body mass index, total cholesterol, and increased fitness and muscle strength with progressive cardiovascular and resistance training for one hour and 15 min three times a week, and a diet with total fat intake less than or equal to 30% of total calories, a saturated fat intake less than or equal to 10% of total calories, and a fiber intake greater than 25 g per day [24]. In contrast, a study of home-based progressive resistance training among HIV-infected women without dietary intervention improved waist circumference and cardiorespiratory fitness, but no effect was seen on blood pressure or lipids [25]. Another study of HIV-infected patients with dyslipidemia receiving a lipid-lowering diet followed 161 patients for 3 months and 70 patients for 6 months, and showed a significant decrease in total cholesterol and triglyceride levels in subjects with 'good' compliance [26]. Such studies suggest the potential utility of a lifestyle modification program among HIV-infected patients with fat redistribution and dyslipidemia. Among non-HIV-infected patients, lifestyle modification in the DPP study improved metabolic parameters among patients with the metabolic syndrome [27]. Although the prevalence of the metabolic syndrome is increased among HIV-infected patients, no study to date has evaluated the effects of a lifestyle modification program in this population, in whom cardiovascular risk is increased [28].
In this study, we investigated whether a lifestyle modification program, which included weekly one-on-one dietary counseling on healthy eating and increasing activity, would improve the NCEP metabolic syndrome criteria and decrease cardiovascular risk in patients with HIV disease. Subjects were randomly assigned to participate in a lifestyle modification program or no intervention over a 6-month period. An advantage of using a metabolic syndrome definition is that it is standardized, easily generalized, and incorporates metabolic as well as anthropometric risk factors, which are easily measured and understood.
Intensive lifestyle modification over 6 months improved waist circumference and systolic blood pressure. Waist circumference decreased significantly more in the lifestyle intervention group compared with the control group. Waist circumference is known to be a significant cardiac risk factor in non-HIV-infected patients [3], and data in HIV-infected patients suggest that central adiposity is associated with significant metabolic abnormalities [10,29]. The change in systolic blood pressure achieved in this study was highly significant, and was similar in magnitude to that achieved in the DASH study of intensive lifestyle modification [30]. Limited treatment strategies have proved successful in the treatment of blood pressure among HIV patients. Lifestyle modification is not associated with the adverse effects of other pharmacological strategies and appears beneficial in this regard. In addition, lifestyle modification was associated with a significant decrease in HbA1C, which may further reduce cardiovascular and diabetes risk in this population. In the DPP study, after which this study was modeled, lifestyle intervention resulted in an approximately similar 0.1% reduction in HbA1C over the initial few months. In longer follow-up, lifestyle intervention prevented an increase in HbA1C compared with the control group [21]. Longer studies in HIV-infected patients are necessary to determine whether the initial changes in CVD risk factors seen in this study are maintained over time.
Waist circumference was higher at baseline in the lifestyle group, but the change in waist circumference over 6 months was significant between the groups in confirmatory analyses using ANCOVA to control for baseline values. In contrast, the activity level reported by the MAQ was higher at baseline in the control group, in whom changes might have reflected a regression toward the mean. However, statistically significant results were obtained comparing changes between the groups. Body mass index also tended to be higher at baseline in the lifestyle group, although this was not statistically significant.
Other traditional markers of cardiovascular risk, including total cholesterol, LDL and HDL-cholesterol, did not improve significantly during the study, which may relate to the pathophysiology of lipid abnormalities in HIV-infected patients [31,32], or an effect of ART that may limit the known effects of lifestyle modification on lipid metabolism. In addition, the relatively small sample size and study duration may have limited our capacity to detect changes in these endpoints. Immune function remained stable and did not change between the groups.
Physical activity is important to prevent chronic diseases including CVD and diabetes, and low levels of physical activity may be considered a risk factor for developing these diseases. Estimates of VO2max using submaximal exercise test data have been used as a surrogate measure of cardiorespiratory fitness and aerobic capacity [33]. The MAQ was designed to assess activity patterns accurately [13], and was used in the DPP study [11]. Levels of physical activity as assessed by the MAQ improved significantly and VO2max tended to improve in the lifestyle modification group.
Treatment with lifestyle modification improved critical indices of cardiovascular risk in HIV-infected patients with the metabolic syndrome. The implementation of lifestyle modification may therefore be a useful strategy in this group of HIV-infected patients. Further studies are needed to assess combined programs with lifestyle modification, progressive resistance training, and lipid-lowering agents to reduce cardiovascular risk further.
Sponsorship: This study was funded by National Institutes of Health grant no. R01 DK-49302 M01-RR-01066, National Institutes of Health, National Centre for Research Resources, General Clinical Research Centres Program.
References
1. McGill HC Jr, McMahan CA, Zieske AW, Malcom GT, Tracy RE, Strong JP. Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation 2001; 103:1546-1550.
[Fulltext Link] [Medline Link] [Context Link]
2. Eberly LE, Stamler J, Neaton JD. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med 2003; 163:1077-1083.
[Medline Link] [CrossRef] [Context Link]
3. Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005; 366:1640-1649.
[Medline Link] [CrossRef] [Context Link]
4. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001; 24:683-689.
[Medline Link] [Context Link]
5. Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998; 12:F51-F58.
[Context Link]
6. Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet 1998; 351:871-875.
[Medline Link] [CrossRef] [Context Link]
7. Saint-Marc T, Partisani M, Poizot-Martin I, Rouviere O, Bruno F, Avellaneda R, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS 2000; 14:37-49.
[Fulltext Link] [Medline Link] [Context Link]
8. Gazzaruso C, Sacchi P, Garzaniti A, Fratino P, Bruno R, Filice G. Prevalence of metabolic syndrome among HIV patients. Diabetes Care 2002; 25:1253-1254.
[Medline Link] [Context Link]
9. Jerico C, Knobel H, Montero M, Ordonez-Llanos J, Guelar A, Gimeno JL, et al. Metabolic syndrome among HIV-infected patients: prevalence, characteristics, and related factors. Diabetes Care 2005; 28:132-137.
[Medline Link] [Context Link]
10. Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis 2001; 32:130-139.
[Medline Link] [CrossRef] [Context Link]
11. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002; 25:2165-2171.
[Context Link]
12. Meininger G, Hadigan C, Rietschel P, Grinspoon S. Body-composition measurements as predictors of glucose and insulin abnormalities in HIV-positive men. Am J Clin Nutr 2002; 76:460-465.
[Medline Link] [Context Link]
13. Kriska A, Knowler W, LaPorte R. Development of a questionnaire to examine the relationship of physical activity and diabetes in Pima Indians. Diabetes Care 1990; 13:401-411.
[Medline Link] [Context Link]
14. American College of Sports Medicine. American College of Sports Medicine's Guidelines for exercise testing and prescription, 5th ed. Baltimore: Williams and Wilkins; 1995.
[Context Link]
15. American College of Sports Medicine. American College of Sports Medicine's Exercise management for persons with chronic diseases and disabilities. Champaign: Human Kinetics; 1997.
[Context Link]
16. Stanforth PR, Ruthven MD, Gagnon J, Bouchard C, Leon AS, Rao DC, et al. Accuracy of prediction equations to estimate submaximal V02 during cycle ergometry: the HERITAGE Family Study. Med Sci Sports Exerc 1999; 31:183-188.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
17. Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: a randomized controlled trial. JAMA 2000; 284:472-477.
[Medline Link] [CrossRef] [Context Link]
18. Dixon WJ, Massey FJ. Introduction to statistical analysis. 2nd ed. New York: McGraw-Hill Book Company; 1957.
[Context Link]
19. Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation 2001; 104:257-262.
[Fulltext Link] [Medline Link] [Context Link]
20. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16-22.
[Medline Link] [CrossRef] [Context Link]
21. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393-403.
[Context Link]
22. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344:1343-1350.
[Medline Link] [CrossRef] [Context Link]
23. McAuley KA, Williams SM, Mann JI, Goulding A, Chisholm A, Wilson N, et al. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes Care 2002; 25:445-452.
[Medline Link] [Context Link]
24. Roubenoff R, Schmitz H, Bairos L, Layne J, Potts E, Cloutier GJ, et al. Reduction of abdominal obesity in lipodystrophy associated with human immunodeficiency virus infection by means of diet and exercise: case report and proof of principle. Clin Infect Dis 2002; 34:390-393.
[Medline Link] [CrossRef] [Context Link]
25. Dolan SE, Frontera W, Librizzi J, Ljungquist K, Juan S, Droman R, et al. The effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med 2006; 166:1225-1231.
[Medline Link] [CrossRef] [Context Link]
26. Barrios A, Blanco F, Garcia-Benayas T, Gomez-Viera JM, de la Cruz JJ, Soriano V, et al. Effect of dietary intervention on highly active antiretroviral therapy-related dyslipemia [see Comment]. AIDS 2002; 16:2079-2081.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
27. Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005; 142:611-619.
[Medline Link] [Context Link]
28. Hadigan C, Meigs JB, Wilson PW, D'Agostino RB, Davis B, Basgoz N, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis 2003; 36:909-916.
[Medline Link] [CrossRef] [Context Link]
29. Dolan SE, Hadigan C, Killilea KM, Sullivan MP, Hemphill L, Lees RS, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr 2005; 39:44-54.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
30. Elmer PJ, Obarzanek E, Vollmer WM, Simons-Morton D, Stevens VJ, Young DR, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med 2006; 144:485-495.
[Medline Link] [Context Link]
31. Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992; 74:1045-1052.
[Medline Link] [CrossRef] [Context Link]
32. Hellerstein MK, Grunfeld C, Wu K, Christiansen M, Kaempfer S, Kletke C, et al. Increased de novo hepatic lipogenesis in human immunodeficiency virus infection. J Clin Endocrinol Metab 1993; 76:559-565.
[Medline Link] [CrossRef] [Context Link]
33. Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA 2005; 294:2981-2988.
[Medline Link] [Context Link]
|
|
| |
| |
|
|
|