| |
Lung Cancer Particularly Aggressive in Patients with HIV
|
| |
| |
By David Douglas
NEW YORK OCT 04, 2006 (Reuters Health) - HIV-infected patients are at increased risk of lung cancer mortality, according to researchers at the Johns Hopkins Medical Institutes, Baltimore. They suggest that physicians maintain a high index of suspicion for lung cancer in HIV-infected smokers.
"At Johns Hopkins," lead investigator Dr. Malcolm V. Brock, told Reuters Health, "we now have about 105 HIV patients with biopsy-proven lung cancer. This is the largest reported cohort in the world from a single institution."
Lung cancer in this population "is aggressive, occurs at a young age -- median age 46 -- and since most patients present with advanced stage, survival is poor," he added.
Dr. Brock and colleagues retrospectively studied data on more than 5,000 patients with lung cancer. Of these, 92 were HIV-positive while the status of the remainder was indeterminate.
Mortality was increased in the HIV group and 92% died of lung cancer (hazard ratio, 1.57), the investigators report in the September issue of the Journal of Acquired Immune Deficiency Syndromes.
Being black and having advanced stage disease was associated with poorer survival, but after adjustment for these factors, HIV infection was not.
In 32 patients followed at the HIV clinic, 60% of chest radiographs had no evidence of neoplasm within 1 year of diagnosis. This was true of only 1 of 28 (4%) CT scans.
"Since lung cancer usually occurs when patients are in their mid-sixties," concluded Dr. Brock, "at our hospital, we are focusing on increasing the awareness among our clinicians in our specialty HIV clinics that young HIV-infected smokers are at risk for this disease."
SOURCE:
J Acquir Immune Defic Syndr 2006;43:47-55
Delayed Diagnosis and Elevated Mortality in an Urban Population With HIV and Lung Cancer: Implications for Patient Care
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 43(1) September 2006 pp 47-55
"....We describe an important association between HIV status and lung cancer survival in a large urban institution. As in smaller series,3-5,17,20,21 the HIV-infected patients presented with more advanced lung cancer than HIV-indeterminate individuals and had a poorer prognosis with a 6-month median survival. This 6-month median survival did not change between the pre-HAART and the HAART eras. Because of the small sample size in previous series, it has been unclear if lung cancer or advanced HIV disease was the cause of high mortality. In reviewing large administrative data sets that lacked detailed clinical data, Biggar et al3 recently reported little change in survival in lung cancer patients with AIDS over the last 2 decades, hypothesizing that lung cancer, rather than HIV infection, dominated survival rate. We determined from our 92 patients that the majority died of their malignancy....
..... given that lung cancer is the most frequent non-AIDS-defining malignancy, occurs at a young age, is rapidly progressive, and has an increased mortality, physicians should have a high clinical suspicion of this disease in HIV-infected smokers and should encourage smoking cessation in all of these patients....
....The clinical implications for physicians caring for HIV-infected patients who are heavy smokers are that frequent clinic visits, complete with chest-specific histories, clinical examinations, and chest radiographs, may be inadequate to detect these lung cancers early. Lung cancer in the HIV-infected patient seems particularly aggressive, but early diagnosis and surgical intervention may enable longer survival."
Authors: Brock, Malcolm V. MD*; Hooker, Craig M. MPH*; Engels, Eric A. MD, MPH* ; Moore, Richard D. MD* ; Gillison, Maura L. MD, PhD* ; Alberg, Anthony J. PhD ; Keruly, Jeanne C. RN*; Yang, Stephen C. MD*; Heitmiller, Richard F. MD* ; Baylin, Stephen B. MD*; Herman, James G. MD*; Brahmer, Julie R. MD*
From the *Johns Hopkins Hospital, Baltimore, MD; Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; and Union Memorial Hospital, Baltimore, MD.
Abstract
Objective: Lung cancer is more common in HIV-infected patients than in the general population. We examined how effectively lung cancer was being diagnosed in our HIV-infected patients.
Methods: Retrospective study assessing clinical diagnosis of lung cancer in HIV-infected patients at Johns Hopkins Hospital between 1986 and 2004.
Results: Ninety-two patients were identified. Compared to HIV-indeterminate patients (n = 4973), HIV-infected individuals were younger with more advanced cancer. CD4 counts and HIV-1 RNA levels indicated preserved immune function. Mortality was higher in HIV-infected patients, with 92% dying of lung cancer (hazard ratio, 1.57; 95% confidence interval, 1.25-1.96), compared to HIV-uninfected patients. Advanced stage and black race were associated with worse survival. After adjustment for these factors, HIV infection was not associated with increased mortality (hazard ratio, 1.04; 95% confidence interval, 0.83-1.32). Of 32 patients followed in our HIV clinic, 60% of chest radiographs had no evidence of neoplasm within 1 year of diagnosis compared to only 1 (4%) of 28 chest computed tomography scans. Nonspecific infiltrates were observed in 9 patients in the same area that cancer was subsequently diagnosed.
Conclusions: HIV-infected lung cancer patients have shortened survival mainly due to advanced stage. Low clinical suspicion and overreliance on chest radiographs hindered earlier detection. Aggressive follow-up of nonspecific pulmonary infiltrates in these patients is warranted.
Background
With the introduction of highly active antiretroviral therapy (HAART) in 1996, the natural history of HIV-1 infection has changed with prolonged survival and fewer opportunistic infections.1 Moreover, the incidence of the most frequent AIDS-related malignancies, Kaposi sarcoma and non-Hodgkin lymphoma, has dramatically declined.2 Lung cancer is the most frequent non-AIDS-related malignancy, and HIV-infected patients are at a 3- to 8-fold increased risk compared with the general population.3-11 In the United States, HIV infection has had a disproportionate effect on racial and ethnic minorities, especially in urban areas with high rates of poverty and substance abuse.12 At the Johns Hopkins Hospital in metropolitan Baltimore, the current incidence of lung cancer in our HIV patients is significantly higher than in the early 1990s, and lung cancer has become an increasingly important cause of morbidity and mortality in our HIV patients.13 In France, lung cancer is now the third leading cause of cancer death in patients with AIDS.14
Most single institutions report few documented HIV-infected lung cancer cases (<25 individuals), and many clinicians remain unaware of any association between the 2 diseases. The clinical and pathologic characteristics of HIV-infected patients with lung cancer are quite consistent, and the natural history of lung cancer in the backdrop of HIV-infection appears to be quite different than in lung cancer patients in general (ie, "HIV indeterminate," presumably uninfected). Most have strong smoking histories, but are younger at presentation than HIV-indeterminate patients, have more advanced stage, and are more likely to have adenocarcinoma cell type with a paucity of small-cell carcinoma.4 Remarkably, at the time of lung cancer diagnosis, these HIV-infected lung cancer patients are typically asymptomatic for their HIV condition and have CD4 cell counts of more than 200 cells/_L.7 Most have low HIV-1 RNA levels and are doing well on HAART, with only a few patients having a history of opportunistic infections, such as oral candidiasis.5 Despite only moderate immunodeficiency, however, the survival rates for HIV-infected lung cancer patients are significantly reduced compared to HIV-indeterminate lung cancer patients.3,4,15-18
In this study, we describe 92 HIV-infected lung cancer patients from the Johns Hopkins Hospital diagnosed between 1986 and 2004. These patients are from hospital-based medical clinics that serve a large urban community. We had a wide range of clinical and pathological variables to elucidate the association of factors most critical to overall survival, and we examined if lung cancer was being effectively detected in our patients with HIV. Importantly, we found that despite our urban patients having frequent clinic visits and regular physical examinations by our physicians, patients still presented with late stage lung cancer and high mortality rates.
METHODS
Study Population
Data were acquired regarding the HIV serostatus of 5065 patients with lung cancer treated at Johns Hopkins Hospital between January 1, 1986, and December 31, 2004. Ninety-two HIV-infected patients with lung cancer were identified. Eighty-six of the 92 patients had their lung cancer specimens diagnosed by a Johns Hopkins pathologist. The remaining 6 patients had extensive medical records detailing precise diagnostic and clinical information concerning their lung cancer. HIV serostatus and transmission, demographics, comorbidities, treatment, survival, and most recent HIV disease markers (absolute/nadir CD4 cell counts, HIV-1 RNA levels) were ascertained using HIV clinic charts. Lung cancer subtypes, staging, performance status, and associated risk factors were obtained from a lung cancer database, clinical charts, the institution's cancer registry, and autopsy reports. Due to the long study period and the dramatic changes induced by HAART, patient data were also analyzed according to whether patients were treated before or after the introduction of HAART (1986-1995 vs 1996-2004, respectively). HIV diagnosis date was either the date of seroconversion at Johns Hopkins or the date of initial enrollment at our institution with a confirmed HIV diagnosis. AIDS determination was made according to the criteria of the Centers for Disease Control.19 Study approval was obtained from our institution's review board.
To evaluate how effectively we diagnosed lung cancer, we studied in more detail the clinical records of 32 of our 92 HIV-infected patients who received nonepisodic longitudinal care in our specialized HIV outpatient clinic (ie, at least 2 clinic visits within the initial year of HIV presentation, and at least 2 clinic visits in the year preceding lung cancer diagnosis). Variables abstracted included dates and reasons for HIV clinic visits, types of treatment, any respiratory symptoms or weight loss antecedent to lung cancer diagnosis, performance status, and medical noncompliance. For chest radiographs and computed tomography (CT) studies, we recorded examination dates, clinical indications, lesions observed, as well as whether studies were interpreted as normal, abnormal but no evidence of cancer, or suspicious for cancer. To ensure accuracy, all records were independently abstracted by 2 reviewers.
Statistical Analysis
The primary end point was mortality, ascertained by the date of last follow-up or date of death. Vital status data were from clinical records, death certificates, or the Social Security Death Index. Detailed inpatient or outpatient clinical records were available in all but 19 patients to ascertain the exact cause of death. Death certificates were the sole means of ascribing cause of death in only 19 patients, and these death certificates were independently reviewed by 2 physicians. Cause of death was classified as cancer related, AIDS related, or neither cancer nor AIDS related. In the HIV-infected group, survival analysis was cancer specific. In the HIV-indeterminate group, survival analysis was for all-cause mortality.
Time to death was modeled using the Kaplan-Meier method, and the association of factors with time to death was analyzed using the Cox proportional hazards model. Results of Cox modeling are reported as hazard ratios with 95% confidence intervals (CIs). In multivariate models, any variable that, by itself, changed the unadjusted hazard ratio by more than 10% was considered a potential confounder.
Comparison of continuous and dichotomous variables was performed using the 2-sample t test and _ 2 test, respectively. Due to incomplete data on the specific treatment of HIV-indeterminate lung cancer patients, we were unable to analyze treatment differences between HIV-infected and HIV- indeterminate lung cancer patients. Associations between continuous variables were measured using the Spearman rank correlation coefficient (_). Results were considered significant for P values less than 0.05. All analyses were accomplished using the Stata statistical software (StataCorp, College Station, TX).
RESULTS
Demographics of Study Individuals
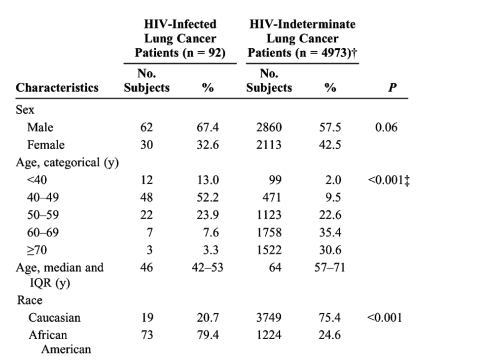
The 92 HIV-infected lung cancer patients identified were younger, more likely to be African American, more frequently presented with adenocarcinoma, and more likely to use illicit injection drugs than HIV-indeterminate patients (Table 1). The younger age at onset may partly explain the lower median smoking pack-years in the HIV-infected lung cancer patients compared to HIV-indeterminate patients (30 vs 50 pack-years, respectively; P < 0.001), despite the smoking prevalence being higher in the former compared to the latter (98.9% vs 88.8%; P = 0.002). Both groups had a majority of men. If HIV-infected individuals were considered in relation to the introduction of HAART, pre-HAART and HAART cohorts were not demographically different (Table 2).
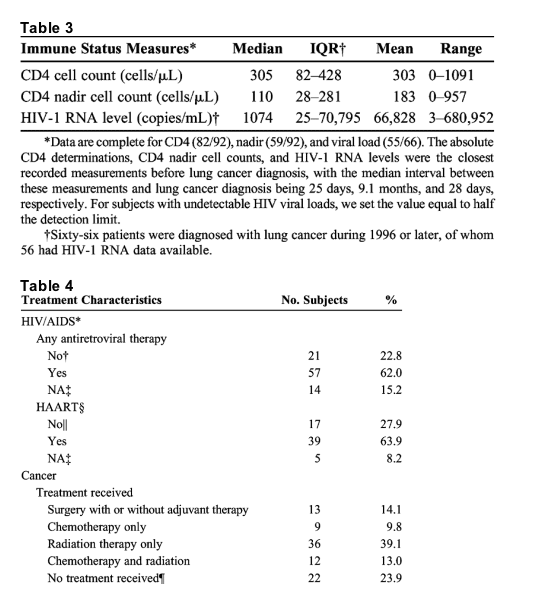
No patient's lung cancer preceded their HIV diagnosis. Three patients had lung cancer detected during the initial evaluation of their newly found HIV disease. The median interval between HIV diagnosis or entry into HIV care and lung cancer was 5.5 years, with only 20% of patients being diagnosed within 2 years of HIV diagnosis. At lung cancer diagnosis, most HIV-infected patients had only moderate T-cell dysfunction, with median CD4 levels of 305 cells/μL. Nadir CD4 levels indicated, however, that many had previously been more immunocompromised (Table 3). The median HIV-1 RNA level was 1074 copies/mL, whereas 18 subjects (27%) had undetectable viral loads. Before lung cancer diagnosis, 62% had taken antiretroviral medication for HIV disease; of those diagnosed from 1996 to 2004, the majority had received HAART (Table 4). Most HIV-infected patients received chemotherapy and/or radiation for their lung cancer. Due to more advanced stage, only 14% underwent surgery with curative intent compared to 27% of HIV-indeterminate patients (P = 0.001; Table 1). Of the HIV-infected group, 24% received no treatment (Table 4).
Stage, Race, Drug Abuse, and Survival
The overall survival from lung cancer was dismal in both HIV-infected and HIV-indeterminate groups, but significantly shorter in HIV-infected patients (median survival: 6.3 vs 9.4 months, respectively; P = 0.002; Fig. 1). Among HIV-infected patients, there was no difference in survival between the pre-HAART and HAART eras (Table 2). Of 78 deceased patients in the HIV-infected group, 73 (93%) had cancer-related deaths, 3 (4%) died of AIDS, and 2(3%) had neither cancer- nor AIDS-related deaths. Only 4 patients were autopsied. Metastatic lung cancer was confirmed in all 4 cases. Coexisting infections (acute bacterial meningitis, mycobacterial infections, and candidiasis) were present in 75% of the autopsied patients.
FIGURE 1. Kaplan-Meier estimates of overall survival rates of HIV-positive lung cancer patients and HIV-indeterminate lung cancer patients at the Johns Hopkins Hospital, according to stages of lung cancer at time of diagnosis. A, The Kaplan-Meier estimates for overall survival indicate that HIV-positive lung cancer patients (n = 92) have a significantly reduced survival rate (1-, 3-, and 5-year survival rate of 27.5%, 11.4%, and 7.6%, respectively) than HIV-indeterminate lung cancer patients (n = 4973), who have a 1-, 3-, and 5-year survival of 47.1%, 23.1%, and 17.2%, respectively (P < 0.001). B, Lung cancer stage in both of these groups is a strong predictor of overall survival. However, compared stage by stage (C-F), the 2 groups have similar survival rates, suggesting that it is the higher proportion of HIV-positive lung cancer patients with stages 3 and 4 of the disease (87% vs 68%, P < 0.001) that contributes to the overall reduced survival. In the HIV-positive group, survival analysis was cancer specific. In the HIV-indeterminate group, survival analysis was for all-cause mortality.
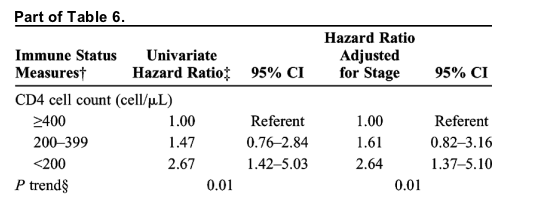
Mortality in the HIV-infected group was 57% greater than in HIV-indeterminate patients (unadjusted hazard ratio, 1.57; 95% CI, 1.25-1.96; Table 5). Importantly, the groups differed by age at onset, race, intravenous drug use, and stage of cancer. We therefore used multivariate Cox modeling to determine independent predictors of mortality after lung cancer. Notably, when we included lung cancer stage as a covariate, the hazard ratio associated with HIV infection decreased to 1.17 (95% CI, 0.94-1.47), indicating that lung cancer stage in both HIV-positive and HIV-indeterminate groups is a strong predictor of overall survival. This dominant effect of lung cancer stage is supported by Kaplan-Meier plots, which showed no substantial differences between HIV-infected and HIV-indeterminate patient survival after stratifying by stage (Fig. 1). Addition of race to the multivariate model further attenuated the effect of HIV infection (adjusted hazard ratio, 1.04; 95% CI, 0.83-1.32; Table 5). None of the other variables significant on univariate analysis explained the increased mortality risk in HIV-infected lung cancer patients, as well as stage and race.
There was minimal correlation between lung cancer stage and either absolute CD4 cell counts (ρ = -0.14) or HIV-1 RNA levels (ρ = -0.16). Although we examined only the cancer-specific mortality among HIV-infected patients, there was still a significant trend for patients with low absolute CD4 cell counts at lung cancer diagnosis to have shortened survival (Table 6). Notably, CD4 cell counts tended to be lower in HIV-infected patients who had no treatment for lung cancer than in those who received cancer therapy (median, 116 vs 314 cells/μL, respectively; P = 0.08).
Low Clinical Suspicion and Delay in Diagnosis
Although most of the 92 patients had been longitudinally followed by physicians at our institution for their HIV disease, 87% presented with advanced lung cancer (stages III and IV), and 69% had distant metastases (stage IV). This compares to 68% HIV-indeterminate patients with advanced lung cancer and 47% with metastases (Table 1).
The 32 patients followed in the HIV clinic had a median of 9.5 clinic visits in the 12 months before lung cancer diagnosis. Demographic and pathological variables, as well as median survival, between our specialty HIV clinic patients and HIV patients seen in other medical clinics in the institution were similar, except that the former were more likely to use illicit drugs (72% vs 50%, respectively; P = 0.04). No patient in our HIV clinic was diagnosed with lung cancer while asymptomatic.
Despite our 32 patients receiving nonepisodic, longitudinal primary care in our HIV specialty clinic with multiple clinic visits, lung cancer was not detected at an early stage. Eighty-eight percent of HIV-infected patients had advanced disease, and 66% had metastases on lung cancer presentation. Seven patients in the HIV clinic had more than a 10% weight loss in the year antecedent to their lung cancer diagnosis, but cancer was not entertained as a possible etiology. Similar to the total group of 92 patients, only 5 patients from the HIV clinic (16%) underwent surgery because of the advanced stage of presentation. In the main, our HIV-infected patients had reasonably well-controlled HIV disease at lung cancer diagnosis, as reflected by CD4 cell counts (median, 261 cells/_L; interquartile range [IQR], 82-484 cells/_L) and HIV-1 RNA levels (median, 320copies/mL; IQR, 25-3267 copies/mL).
All but 2 clinic patients had chest radiographs within 12months of lung cancer diagnosis because of pulmonary complaints. The majority of these chest radiographs (18/30, 60%) were interpreted as either normal (9/30, 30%) or abnormal with nonspecific infiltrates but no evidence of neoplasm (9/30, 30%). Furthermore, 26 patients had at least 1 chest radiograph within 1 month of lung cancer diagnosis, and 31% (8/26) were read as no neoplasm detected. Of the patients with normal chest radiographs within a year of diagnosis, 9 chest x-rays showed clear lung fields, but subsequent chest CT scans (in 2 cases within 24 hours) showed a malignancy. The 9 radiographs with nonspecific infiltrates were initially all thought to be inflammatory. Four of these 9 patients with infiltrates were followed for more than 3 months before any suspicions of malignancy arose. In 6 of the 9 patients with infiltrates, there was a focal component of the infiltrate in the same area that the cancer was subsequently diagnosed. In 1 of these 6 patients, infiltrates were seen on the day of death, and the diagnosis of metastatic lung cancer with pleural implants was made at autopsy. In a second patient, infiltrates were detected on a preoperative chest film, and multiple neoplastic pleural metastases were observed intraoperatively. Chest CT scans were available in 7 of the 9 patients with nonspecific infiltrates. Six of the 7 chest CT scans detected lesions suspicious for malignancy, suggesting that aggressive follow-up of nonspecific infiltrates with more sensitive chest CT scans can be informative about neoplastic disease. Of our 32 HIV-positive clinic patients, 27 patients received chest CT scans at some point in the year before lung cancer diagnosis. In all but 1 patient, a suspicious abnormality thought to be cancer was detected on CT scan.
DISCUSSION
We describe an important association between HIV status and lung cancer survival in a large urban institution. As in smaller series,3-5,17,20,21 the HIV-infected patients presented with more advanced lung cancer than HIV-indeterminate individuals and had a poorer prognosis with a 6-month median survival. This 6-month median survival did not change between the pre-HAART and the HAART eras. Because of the small sample size in previous series, it has been unclear if lung cancer or advanced HIV disease was the cause of high mortality. In reviewing large administrative data sets that lacked detailed clinical data, Biggar et al3 recently reported little change in survival in lung cancer patients with AIDS over the last 2 decades, hypothesizing that lung cancer, rather than HIV infection, dominated survival rate. We determined from our 92 patients that the majority died of their malignancy.
Similarly, due to small sample size, only speculative explanations have been previously offered to account for high mortality rates in HIV-infected lung cancer patients, including aggressive tumor biology,17 advanced stage of presentation,22 suboptimal cancer therapy,4 and AIDS-related deaths.20 In our large cohort of HIV-infected lung cancer patients, it is clear by multivariate analysis that the advanced stage of presentation of lung cancer in these patients is the major influence on survival. Although we were unable to demonstrate if lung cancer treatment differed by stage between HIV-infected and HIV-indeterminate patients, compared stage by stage, the 2 groups had similar survival rates, again confirming that the higher proportion of HIV-positive lung cancer patients with stages 3 and 4 disease (87% vs 68%, P < 0.001) contributed significantly to the overall reduced survival. Both before and after the introduction of HAART, stages 1 and 2 HIV-infected lung cancer patients received surgery with curative intent at the same rate. The low rate of surgery with curative intent in HIV-infected patients (compared to HIV-indeterminate patients) seemed just to reflect their advanced stage of lung cancer diagnosis. The poor correlation between lung cancer stage and HIV immune status also strongly points to advanced lung cancer stage as the most important contributing factor to the high mortality. Finally, it is not surprising that race was influential because African Americans have lower survival rates than whites in both early- and advanced-stage lung cancer, reflecting other factors, such as poorer medical care.23,24 Interestingly, we found no difference in race by stage distribution among the HIV-infected lung cancer patients.
The young age of onset and the advanced clinical stage may reflect a more aggressive lung cancer in HIV-infected patients. Sridhar et al17 hypothesized that HIV-associated lung cancers are exceptionally virulent and evade clinical screening because of their speed of onset and rapid progression. This is supported by Wistuba et al25 who observed increased genomic instability in lung cancers from HIV-infected patients. Indeed, the AIDS-defining malignancies non-Hodgkin lymphoma, Kaposi sarcoma, and cervical neoplasia all have more aggressive phenotypes than their non-HIV counterparts and present as higher-grade tumors with more advanced stage, more rapid progression, and shorter survival.16,26-29 Nonetheless, treatment of the HIV infection in our patients had little effect on survival, with no difference in survival between pre-HAART and HAART years.
We also asked whether a clinical delay in diagnosis or poor screening contributed to the late stage of lung cancer presentation. As in most specialized HIV outpatient clinics, our current practice does not include asymptomatic screening of HIV-infected smokers for lung cancer, chest radiographs are used to evaluate chest-specific complaints. Although radiological features of lung cancer in HIV-infected patients appear to be similar to those in HIV-indeterminate individuals,22,30,31 normal chest radiographs or those with no evidence of malignancy were obtained in more than half and in almost one third of patients within 1 year and 1 month of lung cancer diagnosis, respectively. In 5 patients with clear lung fields on chest radiograph, a subsequent chest CT within 2months revealed a suspicious lesion not present on the initial radiograph. This attests to the increased sensitivity of chest CT scans and to the importance of aggressive clinic follow-up of any patient harboring evidence of a suspected malignancy even if the chest x-ray is clear.
Several patients presented with nonspecific infiltrates on chest radiographs. Often, these infiltrates were not aggressively pursued, presumably because inflammatory or infectious etiologies were thought more likely. Yet, focal infiltrates in 67% of these chest radiographs were in areas of subsequent cancer. Our data suggest that few clues exist on physical examination to arouse clinical suspicion of lung cancer, and that chest radiographs in these patients are often too atypical to be reliably discerned. Others have reported similar delays in diagnoses in HIV-infected patients with peripheral chest lesions.32 This suggests that current clinical practice does not diagnose early lung cancer in HIV-infected patients, yet early detection is critical because of the aggressive nature of this malignancy. Lack of clinical awareness, low suspicion of lung cancer developing in young patients with HIV, as well as an overreliance on chest radiographs are all contributing factors. Therefore, we advocate that if any newly detected infiltrate in an HIV-infected smoker fails to respond promptly to empiric antibiotics, a chest CT scan should be considered. In addition, any new nodule detected by CT in these patients should be aggressively pursued using rigorous empirical guidelines, such as those proposed by the Early Lung Cancer Action Project group33 and the National Lung Cancer Screening Trial.34 These guidelines have been successful in distinguishing benign from malignant masses and include the use of computer-aided detection algorithms and 3-dimensional representations of lesion volume to identify rapidly growing tumors.
The major strength of this analysis is its large sample of HIV-infected lung cancer patients with detailed clinical data and longitudinal follow-up. There are several study limitations. First, it is possible that patients with profound HIV-related immunosuppression received less intense therapy because of poor performance status. We were unable to use scores of functional status, such as the Karnofsky index, because the information requisite for this assessment was not reliably recorded. Second, our study was retrospective, and all data review was performed cognizant of a particular patient's outcome. Third, few patients were autopsied, and it is possible that HIV-associated deaths did occur with physicians misclassifying the causes of mortality in clinical charts and death certificates. Although our nonblinded approach could have introduced bias, independent data abstraction by 2 investigators supports the validity of our findings. Fourth, the frequency of clinic visits in 4973 HIV-indeterminate patients the year before the lung cancer diagnosis proved difficult to assemble, and we do not know if these HIV-indeterminate patients were followed as closely as our HIV-infected clinic patients. Finally, it would have been interesting to compare survival rates of lung cancer patients without HIV seen at our institution who were of similar sociodemographic background as our HIV-infected study patients. However, this was not possible because detailed socioeconomic data and insurance information were unavailable for all 5065 patients over such a long study period.
In summary, given that lung cancer is the most frequent non-AIDS-defining malignancy, occurs at a young age, is rapidly progressive, and has an increased mortality, physicians should have a high clinical suspicion of this disease in HIV-infected smokers and should encourage smoking cessation in all of these patients. We attribute the late stage of presentation to delayed diagnosis and the aggressiveness of lung cancer in these patients. In fact, stage for stage, HIV-infected and uninfected patients have roughly the same survival. Clinicians should therefore consider diagnostic evaluations to detect this disease earlier, such as chest CT scans of any infiltrative lesion persisting in an asymptomatic HIV-infected smoker after an appropriate course of antibiotics. The current reliance on chest-specific symptoms or abnormal findings on chest radiographs to detect lung cancer may lead to delayed diagnosis and shortened survival. The clinical implications for physicians caring for HIV-infected patients who are heavy smokers are that frequent clinic visits, complete with chest-specific histories, clinical examinations, and chest radiographs, may be inadequate to detect these lung cancers early. Lung cancer in the HIV-infected patient seems particularly aggressive, but early diagnosis and surgical intervention may enable longer survival.
REFERENCES
1. Palella FJ Jr, Delaney KM, Moorman AC, et al. HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853-860.
[Medline Link] [CrossRef] [Context Link]
2. Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19:14-19.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
3. Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39:293-299.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
4. Tirelli U, Spina M, Sandri S, et al. The Italian Cooperative Group on AIDS and Tumors. Lung carcinoma in 36 patients with human immunodeficiency virus infection. Cancer. 2000;88:563-569.
[Medline Link] [CrossRef] [Context Link]
5. Bower M, Powles T, Nelson M, et al. HIV-related lung cancer in the era of highly active antiretroviral therapy. AIDS. 2003;17:371-375.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
6. Serraino D, Boschini A, Carrieri P, et al. Cancer risk among men with, or at risk of, HIV infection in southern Europe. AIDS. 2000;14:553-559.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
7. Spano JP, Massiani MA, Bentata M, et al. Lung cancer in patients with HIV infection and review of the literature. Med Oncol. 2004;21:109-115.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
8. Dal Maso L, Polesel J, Serraino D, et al. Lung cancer in persons with AIDS in Italy, 1985-1998. AIDS. 2003;17:2117-2119.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
9. Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447-3453.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
10. Ricaurte JC, Hoerman MF, Nord JA, et al. Lung cancer in HIV-infected patients: a one-year experience. Int J STD AIDS. 2001;12:100-102.
[Medline Link] [CrossRef] [Context Link]
11. Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736-1745.
[Context Link]
12. Cargill VA, Stone VE. HIV/AIDS: a minority health issue. Med Clin North Am. 2005;89:895-912.
[Medline Link] [CrossRef] [Context Link]
13. Engels EA, Brock MV, Chen J, et al. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383-1388.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
14. Lewden C, Salmon D, Morlat P, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121-130.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
15. Chiao EY, Krown SE. Update on non-acquired immunodeficiency syndrome-defining malignancies. Curr Opin Oncol. 2003;15:389-397.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
16. Cooley T. Non-AIDS-defining cancer in HIV-infected people. Hematol Oncol Clin North Am. 2003;17:889-899.
[Medline Link] [CrossRef] [Context Link]
17. Sridhar KS, Flores MR, Raub WA Jr, et al. Lung cancer in patients with human immunodeficiency virus infection compared with historic control subjects. Chest. 1992;102:1704-1708.
[Medline Link] [Context Link]
18. Tenholder MF, Jackson HD. Bronchogenic carcinoma in patients seropositive for human immunodeficiency virus. Chest. 1993;104:1049-1053.
[Medline Link] [Context Link]
19. Castro KG, Ward JW, L Slutsker, et al. Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1-19.
[Context Link]
20. Vyzula R, Remick SC. Lung cancer in patients with HIV-infection. Lung Cancer. 1996;15:325-339.
[Medline Link] [CrossRef] [Context Link]
21. Alshafie MT, Donaldson B, Oluwole SF. Human immunodeficiency virus and lung cancer. Br J Surg. 1997;84:1068-1071.
[Medline Link] [CrossRef] [Context Link]
22. Bazot M, Cadranel J, Khalil A, et al. Computed tomographic diagnosis of bronchogenic carcinoma in HIV-infected patients. Lung Cancer. 2000;28:203-209.
[Medline Link] [CrossRef] [Context Link]
23. Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198-1205.
[Medline Link] [CrossRef] [Context Link]
24. Blackstock AW, Herndon JE II, Paskett ED, et al. Outcomes among African-American/non-African-American patients with advanced non-small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J Natl Cancer Inst. 2002;94:284-290.
[Medline Link] [CrossRef] [Context Link]
25. Wistuba II, Behrens C, Milchgrub S, et al. Comparison of molecular changes in lung cancers in HIV-positive and HIV-indeterminate subjects. JAMA. 1998;279:1554-1559.
[Medline Link] [CrossRef] [Context Link]
26. Levine AM. Acquired immunodeficiency syndrome-related lymphoma. Blood. 1992;80:8-20.
[Medline Link] [Context Link]
27. Friedman-Kien AE, Laubenstein LJ, Rubinstein P, et al. Disseminated Kaposi's sarcoma in homosexual men. Ann Intern Med. 1982;96(6 pt 1):693-700.
[Context Link]
28. Friedman SL, Wright TL, Altman DF. Gastrointestinal Kaposi's sarcoma in patients with acquired immunodeficiency syndrome. Endoscopic and autopsy findings. Gastroenterology. 1985;89:102-108.
[Medline Link] [Context Link]
29. Del Mistro A, Chieco Bianchi L. HPV-related neoplasias in HIV-infected individuals. Eur J Cancer. 2001;37:1227-1235.
[Medline Link] [CrossRef] [Context Link]
30. Braun MA, Killam DA, Remick SC, et al. Lung cancer in patients seropositive for human immunodeficiency virus. Radiology. 1990;175:341-343.
[Medline Link] [Context Link]
31. White CS, Haramati LB, Elder KH, et al. Carcinoma of the lung in HIV-positive patients: findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1995;164:593-597.
[Medline Link] [Context Link]
32. Fishman JE, Schwartz DS, Sais GJ, et al. Bronchogenic carcinoma in HIV-positive patients: findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1995;164:57-61.
[Medline Link] [Context Link]
33. Henschke CI, Yankelevitz DF, McCauley DI, et al. Guidelines for the use of spiral computed tomography in screening for lung cancer. Eur Respir J Suppl. 2003;39:45s-51s.
[Medline Link] [Context Link]
34. Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the lung screening study of the National Cancer Institute. Chest. 2004;126:114-121.
[Medline Link] [CrossRef] [Context Link]
|
|
| |
| |
|
|
|