| |
Fish Intake, Contaminants, and Human Health
|
| |
| |
Evaluating the Risks and the Benefits
Dariush Mozaffarian, MD, DrPH; Eric B. Rimm, ScD
JAMA. Oct 18, 2006;296:1885-1899.
ABSTRACT
Context Fish (finfish or shellfish) may have health benefits and also contain contaminants, resulting in confusion over the role of fish consumption in a healthy diet.
Evidence Acquisition We searched MEDLINE, governmental reports, and meta-analyses, supplemented by hand reviews of references and direct investigator contacts, to identify reports published through April 2006 evaluating (1) intake of fish or fish oil and cardiovascular risk, (2) effects of methylmercury and fish oil on early neurodevelopment, (3) risks of methylmercury for cardiovascular and neurologic outcomes in adults, and (4) health risks of dioxins and polychlorinated biphenyls in fish. We concentrated on studies evaluating risk in humans, focusing on evidence, when available, from randomized trials and large prospective studies. When possible, meta-analyses were performed to characterize benefits and risks most precisely.
Evidence Synthesis Modest consumption of fish (eg, 1-2 servings/wk), especially species higher in the n-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), reduces risk of coronary death by 36% (95% confidence interval, 20%-50%; P<.001) and total mortality by 17% (95% confidence interval, 0%-32%; P = .046) and may favorably affect other clinical outcomes. Intake of 250 mg/d of EPA and DHA appears sufficient for primary prevention. DHA appears beneficial for, and low-level methylmercury may adversely affect, early neurodevelopment. Women of childbearing age and nursing mothers should consume 2 seafood servings/wk, limiting intake of selected species. Health effects of low-level methylmercury in adults are not clearly established; methylmercury may modestly decrease the cardiovascular benefits of fish intake. A variety of seafood should be consumed; individuals with very high consumption (5 servings/wk) should limit intake of species highest in mercury levels. Levels of dioxins and polychlorinated biphenyls in fish are low, and potential carcinogenic and other effects are outweighed by potential benefits of fish intake and should have little impact on choices or consumption of seafood (women of childbearing age should consult regional advisories for locally caught freshwater fish).
Conclusions For major health outcomes among adults, based on both the strength of the evidence and the potential magnitudes of effect, the benefits of fish intake exceed the potential risks. For women of childbearing age, benefits of modest fish intake, excepting a few selected species, also outweigh risks.
Author Conclusion: Potential risks of fish intake must be considered in the context of potential benefits. Based on strength of evidence and potential magnitudes of effect, the benefits of modest fish consumption (1-2 servings/wk) outweigh the risks among adults and, excepting a few selected fish species, among women of childbearing age. Avoidance of modest fish consumption due to confusion regarding risks and benefits could result in thousands of excess CHD deaths annually and suboptimal neurodevelopment in children.
Comparing different types of fish, lower risk of cardiovascular heart disease appears more strongly related to intake of oily fish (eg, salmon, herring, sardines), rather than lean fish (eg, cod, catfish, halibut).10, 15 Fish intake may modestly affect other cardiovascular outcomes, but evidence is not as robust as for CHD death.
For all ages evaluated (25-34 to 85 years), CHD benefits outweighed cancer risks by 100- to 370-fold for farmed salmon and by 300- to more than 1000-fold for wild salmon.
While further research is needed, it appears unlikely that most commercially prepared fried fish meals lower cardiovascular risk. Commercially-prepared fried fish meals from fast food restaurants or supermarket frozen sections123-124 are often made using white-meat fish (lower in n-3 PUFAs)27, 123 and prepared with partially hydrogenated oils (containing trans fats) or oils reused for multiple frying cycles (introducing oxidative/deteriorative products191). Higher cardiovascular risk seen with fried fish intake.
Supplements. Fish oil capsules contain 20% to 80% of EPA and DHA by weight (200-800 mg/g185-186), little to no mercury,187 and variable levels of PCBs (0-450 ng/g,116, 188) and dioxins (0.2-11 TEQ pg/g114, 189). Given small amounts of fish oil consumed (1-3 g/d), exposure to PCBs and dioxins from fish oil intake is low. "Functional foods" supplemented with EPA and DHA (eg, dairy products, salad dressings, cereals) can also provide reasonable intake to individuals not consuming seafood.190 Compared with supplements, fish intake also provides potentially beneficial protein, vitamin D, and selenium.121
Optimal Intakes
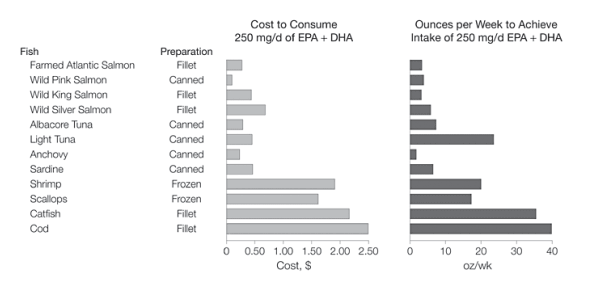
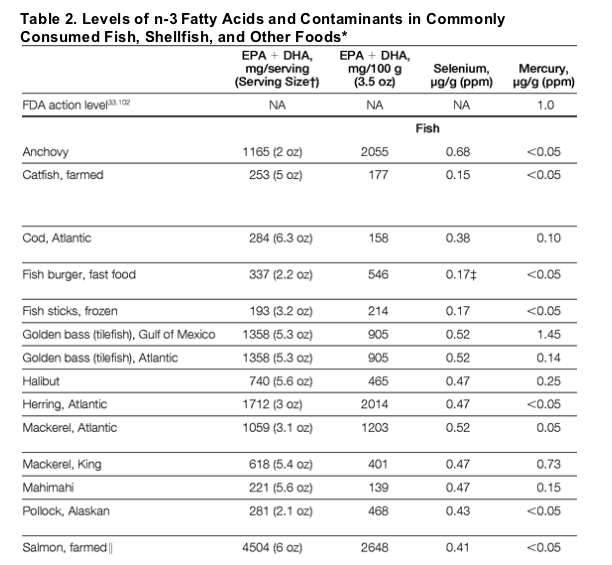
Optimal intake of n-3 PUFAs may vary depending on population and outcome of interest. In the general population, 250 mg/d of EPA and DHA is a reasonable target intake to reduce CHD mortality. Because dietary n-3 PUFAs persist for weeks in tissue membranes,203 this can be converted to a weekly intake of 1500-2000 mg. This corresponds to one 6-oz serving/wk of wild salmon or similar oily fish, or more frequent intake of smaller or less n-3 PUFA-rich servings (Table 2). For individuals with CHD, 1000 mg/d of EPA and DHA is currently recommended to reduce CHD mortality.204-205 Our analysis suggests that lower doses may be sufficient, but given this population's higher risk and that most data are from primary prevention studies, a target intake of 500 to 1000 mg/d-consistent with the largest secondary prevention trial to date9-appears reasonable. This could be approximated by one 6-oz serving/wk of fish richest in n-3 PUFAs (eg, farmed salmon, anchovies, herring), more frequent consumption of other fish (Table 2), or supplements. Optimal intake levels for other clinical outcomes are not well established.
The effects, if any, of low-level methylmercury exposure in adults are not established; mercury may modestly reduce the cardiovascular benefits of fish intake. One can minimize concerns by choosing fish higher in n-3 PUFAs and lower in mercury or by simply consuming a variety of different seafood. Individuals with high consumption (5 servings/wk) should limit intake of selected species highest in mercury (Table 2).
DHA appears important for early neurodevelopment. Women who are or may become pregnant and nursing mothers should avoid selected species (shark, swordfish, golden bass, and king mackerel; locally caught fish per local advisories) and limit intake of albacore tuna (6 oz/wk) to minimize methylmercury exposure.31, 142 However, emphasis must also be placed on adequate consumption-12 oz/wk-of other fish and shellfish to provide reasonable amounts of DHA31, 142 and avoid further decreases in already low seafood intake among women (74% of women of childbearing age and 85% of pregnant women consume <6 oz/wk).206-207
Continued efforts to limit environmental contamination from organochlorine compounds are appropriate. However, levels of PCBs and dioxins in fish are low, similar to those in several other foods, and the magnitudes of possible risks in adults are greatly exceeded by benefits of fish intake and should have little impact on individual decisions regarding fish consumption (for locally caught freshwater fish, women of childbearing age should consult regional advisories).
INTRODUCTION
Since the publication of pioneering studies demonstrating low rates of death from coronary heart disease (CHD) among Greenland Eskimos,1 fish (used herein to refer to finfish or shellfish) has been considered a healthy food. During ensuing years, evidence from several research paradigms-including animal-experimental, observational, and clinical studies-further supported this hypothesis and identified 2 long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as the likely active constituents.2-20 DHA also appears important for neurodevelopment during gestation and infancy.21-26 Conversely, concern has arisen over potential harm from mercury, dioxins, and polychlorinated biphenyls (PCBs) present in some fish species.27-34 The public is faced with seemingly conflicting reports on the risks and benefits of fish intake, resulting in controversy and confusion over the role of fish consumption in a healthy diet.35-36 To elucidate the relative risks and benefits, we reviewed the scientific evidence for adverse and beneficial health effects of fish consumption.
EVIDENCE ACQUISITION
Identification of Studies
A myriad of exposures and outcomes have been related to fish consumption; we focused on populations and topics for which evidence and concern are greatest. We searched MEDLINE, governmental reports, and systematic reviews and meta-analyses to identify reports published through April 2006 evaluating (1) intake of fish or fish oil and risk of cardiovascular events and mortality, (2) effects of methylmercury and fish oil on early neurodevelopment, (3) risks of methylmercury for cardiovascular and neurologic outcomes in adults, and (4) health risks of dioxins and PCBs in fish.
MEDLINE search terms were (Fish or n-3 PUFA or omega-3) and (coronary or cardiac or cardiovascular or mortality) and (clinical trial or prospective or meta-analysis); (fish or n-3 PUFA or omega-3 or docosahexaenoic or mercury or methylmercury) and (cognitive or neurologic or neurodevelopment) and (clinical trial or prospective or meta-analysis); (mercury or methylmercury) and (coronary or cardiac or cardiovascular or cognition or neurologic) and (clinical trial or prospective or meta-analysis); (dioxin or polychlorinated biphenyl or PCB) and (fish or seafood). MEDLINE searches were restricted to identify only English-language reports, studies in humans, and adult or child populations (as appropriate) and were supplemented by searches of related articles of relevant identified manuscripts as well as by hand reviews of references from identified reports and direct contact with investigators.
Study Selection
One author (D.M.) screened all identified studies, and the final articles included were selected by both authors by consensus. Because fish intake is related to exposure to many different compounds, including n-3 PUFAs, mercury, and PCBs and dioxins, as well as to multiple different health outcomes, including cardiovascular diseases, neurologic outcomes, and cancer, a systematic quantitative review of every possible combination was beyond the constraints of this report. We concentrated on studies evaluating or estimating risk in humans, focusing on the evidence, when available, from randomized clinical trials and large prospective studies. Metabolic studies and animal-experimental evidence were also considered to elucidate potential mechanisms of effect. The evidence for risks and benefits was considered overall and among different at-risk populations. When possible, pooled or meta-analyses were performed to characterize effects most precisely.37-39 Other potential benefits of fish intake (eg, for cognitive decline or dementia,40 depression or neuropsychiatric disorders,41-42 and asthma or inflammatory disorders43-44) were not reviewed in this report.
EVIDENCE SYNTHESIS
Benefits of Fish Intake
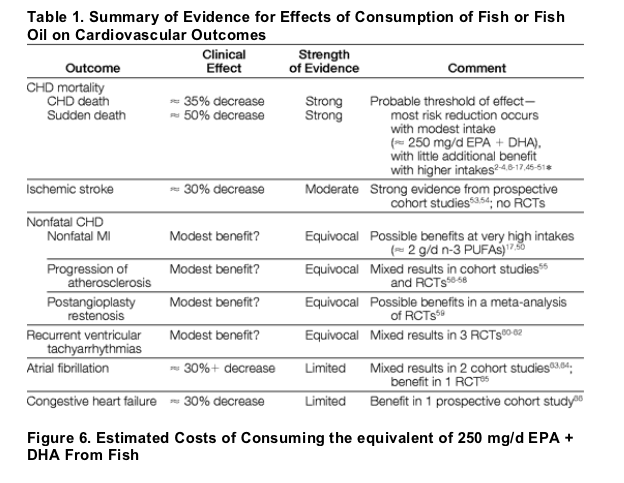
Cardiovascular Outcomes. Death from CHD-ie, documented or suspected fatal myocardial infarction-and sudden death-ie, a sudden pulseless condition of presumed cardiac etiology-are clinically defined entities often sharing the final common pathway of ventricular arrhythmia, often ischemia-induced ventricular fibrillation. The evidence from prospective studies and randomized trials2-4,6-17,45-51 suggests that consumption of fish or fish oil lowers risk of CHD death and sudden death (Figure 1 and Figure 2). Across different studies (Figure 1), compared with little or no intake, modest consumption ( 250-500 mg/d of EPA and DHA) lowers relative risk by 25% or more. Higher intakes do not substantially further lower CHD mortality, suggesting a threshold of effect.52 Pooling all studies, this pattern was clearly evident (Figure 2). At intakes up to 250 mg/d, the relative risk of CHD death was 14.6% lower (95% confidence interval [CI], 8% to 21%) per each 100 mg/d of EPA and DHA, for a total risk reduction of 36% (95% CI, 20% to 50%). At higher intakes, little additional risk reduction was present (0.0% change per each 100 mg/d; 95% CI, -0.9% to +0.8%). This threshold effect explains findings among Japanese populations,17, 50 in whom high background fish intake (eg, median 900 mg/d of EPA and DHA50) is associated with very low CHD death rates (eg, 87% lower than comparable Western populations9, 17), and additional n-3 PUFA intake predicts little further reduction in CHD death; thus, most of the population is already above the threshold for maximum mortality benefits. Comparing different types of fish, lower risk appears more strongly related to intake of oily fish (eg, salmon, herring, sardines), rather than lean fish (eg, cod, catfish, halibut).10, 15 Fish intake may modestly affect other cardiovascular outcomes, but evidence is not as robust as for CHD death (Table 1).17, 50, 53-66
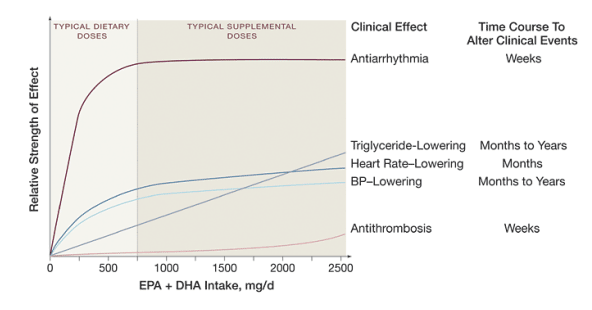
n-3 PUFAs influence several cardiovascular risk factors.18-19,43, 49-50,60-75,79-84 Effects occur within weeks of intake and may result from altered membrane fluidity and receptor responses following incorporation of n-3 PUFAs into cell membranes76-77 and direct binding of n-3 PUFAs to intracellular receptors regulating gene transcription.78 The heterogeneity of the effects of fish or fish oil intake on cardiovascular outcomes is likely related to varying dose and time responses of effects on the risk factors (Figure 3). At typical dietary intakes, antiarrhythmic effects predominate, reducing risk of sudden death and CHD death within weeks. At higher doses, maximum antiarrhythmic effects have been achieved, but other physiologic effects may modestly impact other clinical outcomes (possibly requiring years to produce clinical benefits). For instance, nonfatal myocardial infarction may not be significantly affected by lower doses or shorter durations of intake but may be modestly reduced by higher doses or prolonged intake (eg, 1.8 g/d for 5 years17).
Figure 3. Schema of Potential Dose Responses and Time Courses for Altering Clinical Events of Physiologic Effects of Fish or Fish Oil Intake
The relative strength of effect is estimated from effects of eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) on each risk factor and on the corresponding impact on cardiovascular risk.70-72,79-84 For example, dose response for antiarrhythmic effects is initially steep with a subsequent plateau, and clinical benefits may occur within weeks, while dose response for triglyceride effects is more gradual and monotonic, and clinical benefits may require years of intake. At typical Western levels of intake (eg, <750 mg/d EPA + DHA), the physiologic effects most likely to account for clinical cardiovascular benefits include (1) modulation of myocardial sodium and calcium ion channels, reducing susceptibility to ischemia-induced arrhythmia;18-19 and (2) reduced left ventricular workload and improved myocardial efficiency as a result of reduced heart rate, lower systemic vascular resistance, and improved diastolic filling.67-72,80 At higher levels of intake seen with fish oil supplementation or in Japanese populations49-50 (>750 mg/d EPA + DHA), maximum antiarrythmic effects have been achieved and clinically relevant effects occur on levels of serum triglycerides79 and possibly, at very high doses, thrombosis.75 Potentially important effects on endothelial,73 autonomic,74 and inflammatory43 responses are not shown because dose responses and time courses of such effects on clinical risk are not well established. Effects are not necessarily exclusive: eg, antiarrythmic effects may be partly mediated by effects on blood pressure (BP) or heart rate.
Heterogeneity of clinical effects may also be related to differing pathophysiologies of the clinical outcomes. For instance, disparate pathophysiologies of primary ventricular fibrillation (often ischemia-induced) vs recurrent ventricular tachyarrhythmias (ectopic or reentrant) may explain stronger effects of n-3 PUFAs on the former. Similarly, biological differences in development of atherosclerosis vs acute plaque rupture/thrombosis vs arrhythmia would account for heterogeneous effects of n-3 PUFAs on plaque progression vs nonfatal myocardial infarction vs CHD death. Consumption of fish may displace that of other foods, such as meats or dairy products, in the diet. However, this likely accounts for little of the observed health benefits, because foods replaced would be highly variable among individuals and across cultures, and modest intake of such foods is not associated with CHD risk.85
Total Mortality. n-3 PUFAs most strongly affect CHD death5, 9, 14-16,18 and are unlikely to affect appreciably other causes of mortality. Effects on total mortality in a population would therefore depend on the proportion of deaths due to CHD, ranging from one quarter of deaths in middle-age populations86 to one half of deaths in populations with established CHD.9 Thus, given a 36% reduction in CHD death (Figure 2), intake of fish or fish oil would reduce total mortality by between 9% (36% reduction x 25% CHD deaths) to 18% (36% reduction x 50% CHD deaths), or an average of 14% in mixed populations. This is consistent with a meta-analysis of randomized trials through 20033, 9, 51, 56-57,87-93 that found a nonsignificant 14% reduction in total mortality with n-3 PUFAs (pooled relative risk, 0.86; 95% CI, 0.70 to 1.04).94 When we added additional placebo-controlled, double-blind, randomized trials60-62 performed since 2003, marine n-3 PUFAs reduced total mortality by 17% (pooled relative risk, 0.83; 95% CI, 0.68 to 1.00; P = .046) (Figure 4). This can be compared to effects of statins on total mortality-a 15% reduction-in a meta-analysis of randomized trials (pooled relative risk, 0.85; 95% CI, 0.79 to 0.92).95
Neurologic Development. DHA is preferentially incorporated into the rapidly developing brain during gestation and the first 2 years of infancy, concentrating in gray matter and retinal membranes.26 Infants can convert shorter-chain n-3 fatty acids to DHA,96 but it is unknown whether such conversion is adequate for the developing brain in the absence of maternal intake of DHA.22, 25
Effects of maternal DHA consumption on neurodevelopment have been investigated in observational studies and randomized trials, with heterogeneity in assessed outcomes (visual acuity, global cognition, specific neurologic domains) and timing of DHA intake (gestational vs nursing). In a meta-analysis of 14 trials, DHA supplementation improved visual acuity in a dose-dependent manner.23 Results for cognitive testing are less consistent, possibly due to differences in neurologic domains evaluated21, 25-26; a quantitative pooled analysis of 8 trials estimated that increasing maternal intake of DHA by 100 mg/d increased child IQ by 0.13 points (95% CI, 0.08 to 0.18).24 Most trials evaluated effects of maternal DHA intake during nursing, rather than pregnancy. In a trial among 341 pregnant women, treatment with cod liver oil from week 18 until 3 months postpartum increased DHA levels in cord blood by 50% and raised mental processing scores, a measure of intelligence, at age 4 years.97 This is consistent with observational studies showing positive associations between maternal DHA levels or fish intake during pregnancy and behavioral attention scores, visual recognition memory, and language comprehension in infancy.98-100 Thus, while dose responses and specific effects require further investigation, these studies together indicate that maternal intake of DHA is beneficial for early neurodevelopment.
Risks of Mercury
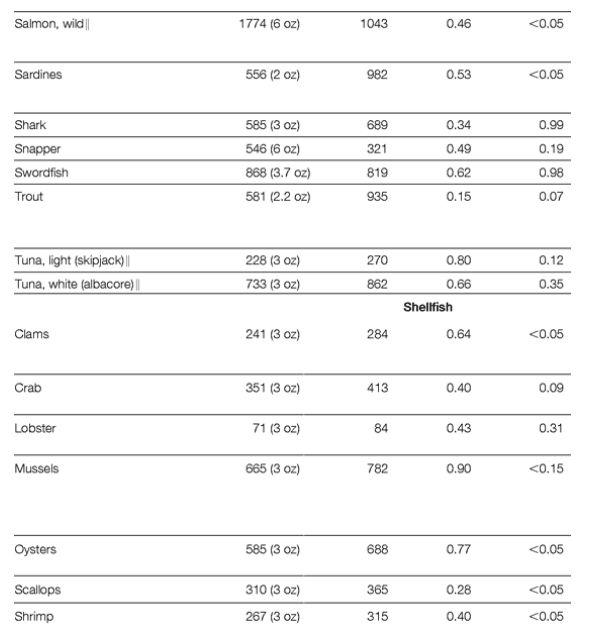
Mercury is a reactive heavy metal emitted from natural sources (volcanoes) and human sources (coal-fired electric power plants, gold mining, institutional boilers, chlorine production, and waste incineration).101 From the atmosphere, mercury cycles from rainwater into lakes and oceans, where it is converted by microbial activity into organic methylmercury. Inorganic mercury is poorly absorbed following ingestion, and elemental mercury does not readily cross tissue barriers. In contrast, methylmercury is readily absorbed and actively transported into tissues.27 Thus, methylmercury bioaccumulates in aquatic food chains and has greater potential toxicity than inorganic mercury.27-28,30 Concentrations of methylmercury in aquatic species depend on levels of environmental contamination and on the predatory nature and lifespan of the species. Larger, longer-living predators (eg, swordfish, shark) have higher tissue concentrations, while smaller or shorter-lived species (eg, shellfish, salmon) have very low concentrations (Table 2).122 Preparation methods have little impact on methylmercury content.27
Health effects of very high mercury exposure following occupational or industrial accidents are well documented, including paresthesias, ataxia, and sensory abnormalities in adults, and delayed cognitive and neuromuscular development following in utero exposure.27, 131 Toxicity appears related to binding of methylmercury to sulfhydryl groups of enzymes, ion channels, and receptors, resulting in inhibition of antioxidant systems and production of free radicals and reactive oxygen species.27, 29 Health effects of chronic low-level mercury exposure-ie, that seen with fish consumption-are less well established. The public is aware of the potential harm from mercury in fish but lacks clear understanding of who is at risk or which seafood species contain mercury.35-36 We review the evidence for health effects below.
Methylmercury and Neurodevelopment
Methylmercury crosses the placenta, and fetal exposure correlates with maternal exposure.132 Marked neurodevelopmental abnormalities occur in children following very high gestational exposure,27, 131 such as from maternal consumption of highly contaminated fish (10-30 ppm mercury) from industrially polluted Minimata Bay, Japan, in the 1950s, or of contaminated grain in Iraq in 1971 (maternal intake, 710-5700 ug/kg per day; 18-598 ppm mercury in maternal hair). More typical methyl mercury exposures are substantially lower: among US women of childbearing age, median (10th-95th percentiles) levels of mercury in hair were 0.19 (0.04-1.73) ppm overall and 0.34 (0.09-2.75) ppm among women consuming 3 or more servings of fish per month.133 These exposure levels do not produce symptomatic neurodevelopmental deficits, but several prospective studies have evaluated whether subclinical effects, detectable with specialized testing, might occur.98, 100, 134-140 Among children from the Faroe Islands,134-135 New Zealand,136-137 and Poland,138 higher gestational exposure to mercury was associated with lower scores on some neurologic tests (eg, finger tapping, naming tests) but not others. In contrast, higher gestational exposure to mercury was associated with higher scores on some neurologic tests among Seychellois children.139-140 In a US cohort, gestational maternal fish intake was positively associated with, but mercury levels in hair were negatively associated with, visual recognition memory scores in infancy,98 indicating possible opposing effects of overall fish consumption (ie, providing DHA) and methylmercury exposure. In a British cohort, gestational mercury exposure was not associated with, but maternal and infant fish intake was associated with, improved neurodevelopmental scores.100 Other studies did not detect consistent associations between gestational exposure to mercury and neurologic test scores during childhood.141
Comparisons across studies are limited by heterogeneity of study designs (prospective vs cross-sectional), mercury assessment methods, neurologic tests used, timing of assessment (infancy vs childhood), and statistical methods. Some analyses are also limited by multiple statistical testing (eg, 30 neurologic variables) or incomplete adjustment for other potential risk factors. Randomized trials to test effects of reducing low-level methylmercury exposure during gestation have not been performed. Nevertheless, given associations with some lower neurologic test scores in some studies, and clinical neurotoxicity of methylmercury following high-level accidental exposures, it is prudent to conclude that subclinical neurodevelopmental deficits may occur at lower exposure levels.
Based on this, the Environmental Protection Agency determined a reference dose, ie, the allowable upper limit of daily intake, for methylmercury of 0.1 ug/kg per day ( 50 μg/wk for a 70-kg woman, calculated from the lower 95% confidence limit at which gestational exposure to mercury may produce abnormal neurologic test scores, multiplied by a 10-fold uncertainty factor)132 and published a focused advisory for women of childbearing age, nursing mothers, and young children.142 The advisory specifically advises such individuals to avoid shark, swordfish, golden bass, and king mackerel (each containing >50 μg methylmercury per serving) (Table 2); to eat up to 12 oz/wk (2 average meals) of a variety of fish and shellfish lower in mercury, including up to 6 oz/wk of albacore tuna (30 μg methylmercury per serving); and to consult local advisories for locally caught freshwater fish. This advisory was not intended for the general population, because the importance of this reference dose to health effects in adults was unclear.143 We review the evidence for such effects below.
Health Effects of Methylmercury in Adults
Cardiovascular Disease. Several studies144-148 have evaluated the relationship between mercury exposure and incidence of cardiovascular disease (Figure 5). The conflicting results provide inconclusive evidence for cardiovascular toxicity of mercury. Notably, in the 2 studies observing higher risk with higher mercury levels, the net effect of fish consumption was still beneficial: greater mercury exposure lessened the benefit associated with consumption of fish or n-3 PUFAs but did not increase overall risk.146, 148, 150 Thus, the principal question may not be whether consumption of mercury-containing fish increases cardiovascular risk but whether consumption of such fish would decrease risk even further if mercury were not present. This would be most true for oily fish species containing higher amounts of n-3 PUFAs (ie, most mercury-containing ocean fish), compared with lean freshwater fish. This is an important public health issue, which requires balancing potentially attenuated benefits of fish intake due to presence of mercury with the costs and practicality of reducing mercury contamination in fish species. Nevertheless, this should not obscure evidence for net cardiovascular benefits of fish consumption, particularly fish richer in n-3 PUFAs.
Neurologic Outcomes. Very high methylmercury exposure from accidents (eg, Minimata)27, 151 or prolonged high intakes of mercury-containing fish (eg, 1-2 fish servings/d, including species high in mercury, for >10 years152) can produce sensorimotor symptoms in adults, most commonly paresthesias, which are often reversible when mercury exposure is reduced. Whether lower exposures produce neurologic abnormalities in adults is not clear.Cross-sectional studies have evaluated associations between mercury levels in hair or blood and subclinical neurologic function in adults. Among Amazon basin and Quebec Cree individuals, both positive and inverse associations were seen between mercury levels and some neurologic measures,153-155 but findings were limited by minimal adjustment for other risk factors and multiple testing (typically 20-30 neurologic tests or participant subgroups). Among US adults, mercury levels were associated with lower visual memory scores (P = .01) but better motor and manual dexterity scores (P = .02) among 20 different outcomes evaluated.156 Among elderly Swedish adults, no associations were found between mercury levels and cognitive function.157 Thus, it is unclear whether low-level methylmercury affects subclinical neurologic outcomes in adults and, if so, what quantities or durations of exposure are necessary. Conversely, a growing body of evidence suggests that fish consumption may favorably affect clinical neurologic outcomes in adults, including ischemic stroke,53 cognitive decline and dementia,40 and depression and other neuropsychiatric disorders.41-42
Possible Mercury-Selenium Interaction. Health effects of mercury may partly result from selenoprotein inactivation, which might be mitigated by adequate intake of selenium, an essential dietary trace element.158-161 Selenium also may reduce tissue accumulation of mercury in fish162 and humans.163 Seafood species are rich dietary sources of selenium.121 A protective effect of selenium may partly account for conflicting results of studies of mercury exposure and neurodevelopmental indices in children160 and of mercury exposure and risk of CHD.164 A potential selenium-mercury interaction would have important public health implications, and additional investigation is warranted.
Risks of PCBs and Dioxins
PCBs are synthetic organochlorine compounds previously used in industrial and commercial processes.34 Dioxins-commonly referring to dibenzodioxins and dibenzofurans-are organochlorine by-products of waste incineration, paper bleaching, pesticide production, and production of polyvinyl chloride plastics.33 Manufacture and processing of PCBs was prohibited in 1977,34 and regulatory and industry efforts have reduced dioxin emissions by more than 90% since 1987.33 Nevertheless, these contaminants persist for long periods in the environment, and thus while levels are steadily declining,33, 110, 112, 127-128 PCBs and dioxins continue to be present in low concentrations in many foods (Table 2).
Cancer Risks. Animal experiments and some evidence in humans indicate that PCBs and dioxins are carcinogenic, possibly related to effects on the aryl hydrocarbon receptor, a transcription factor affecting gene expression.32, 165 Multiple congeners (structural variants) of PCBs and dioxins exist. Potential toxicities of foods are calculated using toxic equivalence (TEQ): the sum of each congener's level in the food multiplied by that congener's toxic equivalency factor (standardized against 2,3,7,8-tetrachlorodibenzo-p-dioxin). In the United States, PCBs comprise 28% and dioxins 72% of total TEQ exposure.120 Among adults, major dietary sources of PCBs and dioxins are beef, chicken, and pork (34% of total TEQ); dairy products (30%); vegetables (22%); fish and shellfish (9%); and eggs (5%).120 Dietary sources are similar for children.120
Although major sources of exposure to PCBs and dioxins are meats, dairy products, and vegetables, considerable attention has been given to fish sources (Table 2). When PCBs and dioxins were measured in farmed and wild salmon,115, 166 levels were similar to those in several other foods (Table 2). Farmed and wild salmon also contained substantial levels of n-3 PUFAs: 4504 and 1774 mg of EPA and DHA per 6 oz, respectively.166 Cancer risks and CHD benefits were evaluated in a quantitative risk-benefit analysis, assuming regular farmed or wild salmon intake to provide 1000 mg/d of EPA and DHA over a 70-year lifetime.167-168 Per 100 000 individuals, consumption of farmed vs wild salmon would result in 24 vs 8 excess cancer deaths, respectively, while consumption of either farmed or wild salmon would result in 7125 fewer CHD deaths.167 We further evaluated age-specific estimates, based on allocation of lifetime cancer risks167 (adjusted for competing risks) by age-specific cancer mortality169 and 25% reduction in age-specific CHD mortality.169 For all ages evaluated (25-34 to 85 years), CHD benefits outweighed cancer risks by 100- to 370-fold for farmed salmon and by 300- to more than 1000-fold for wild salmon.
Notably, estimated CHD benefits are based on prospective studies and randomized trials in humans (Figures 1 and 2); estimated cancer risks include a 10-fold safety factor and are based on animal-experimental data and limited studies in humans at high doses.168 Cancer estimates also assumed lifetime salmon consumption to provide 1000 mg/d of EPA and DHA (eg, four 6-oz servings of wild salmon every week for 70 years). However, CHD mortality reduction may be achieved with lower intake: 250 mg/d (Figures 1 and 2), or one 6-oz wild salmon serving per week. At this intake, CHD benefits would be largely unchanged ( 7125 fewer CHD deaths), while lifetime cancer risk would decrease by 75% (6 and 2 estimated deaths per 100 000 lifetimes for farmed and wild salmon, respectively). Consistent with these very low cancer risks, prospective studies in humans have seen little evidence for effects of fish intake on cancer risk.170
Other Risks. PCBs and dioxins may have noncancer risks in adults, such as immune system or neurologic effects.32-34 Conversely, fish consumption may also have other benefits, possibly lowering risk of other cardiovascular outcomes (Table 1), dementia,40 neuropsychiatric disorders,41-42 and inflammatory disorders.43-44 If present, such additional possible risks would have to exceed additional possible benefits by more than 100-fold to meaningfully alter the present estimates of risks vs benefits. PCB content in fish can be reduced 12% to 40% by trimming belly and back fat during filleting and by not consuming the skin.130 Also, contaminant levels are typically measured in unprepared foods, and cooking may reduce PCB and dioxin content.106
Prenatal (but not postnatal) exposure to PCBs and dioxins has been associated with childhood neurodevelopmental deficits in several,171-177 though not all,178-179 studies. Because most exposure (>90%) generally comes from meat, dairy, and vegetable sources,120, 180 this concern is not specific to fish consumption, particularly since fish also contains potentially beneficial DHA. However, women consuming 1 or more servings/d of commercial freshwater fish or consuming locally caught freshwater fish from highly contaminated inland sources may be more greatly exposed to PCBs and dioxins180 and should consult regional advisories.
Related Considerationsz
Costs. We evaluated potential costs of consuming 250 mg/d of EPA and DHA from fish (Figure 6). The daily cost was as low as 9 cents, or 63 cents/wk. For combinations of different types of salmon; salmon and tuna; or salmon, tuna, anchovies, and sardines, the average cost was 37 cents/d ($2.59/wk) or less. Actual (net) costs would be lower because intake of fish would replace intake of other foods.
Supplements. Fish oil capsules contain 20% to 80% of EPA and DHA by weight (200-800 mg/g185-186), little to no mercury,187 and variable levels of PCBs (0-450 ng/g,116, 188) and dioxins (0.2-11 TEQ pg/g114, 189). Given small amounts of fish oil consumed (1-3 g/d), exposure to PCBs and dioxins from fish oil intake is low. "Functional foods" supplemented with EPA and DHA (eg, dairy products, salad dressings, cereals) can also provide reasonable intake to individuals not consuming seafood.190 Compared with supplements, fish intake also provides potentially beneficial protein, vitamin D, and selenium.121
Commercial Preparation. Commercially-prepared fried fish meals from fast food restaurants or supermarket frozen sections123-124 are often made using white-meat fish (lower in n-3 PUFAs)27, 123 and prepared with partially hydrogenated oils (containing trans fats) or oils reused for multiple frying cycles (introducing oxidative/deteriorative products191). Higher cardiovascular risk seen with fried fish intake15, 54, 63, 66 may relate to this unfavorable balance of benefit vs harm (lower levels of EPA and DHA; higher levels of trans fats/deteriorative products) or to residual confounding from other lifestyle factors. While further research is needed, it appears unlikely that most commercially prepared fried fish meals lower cardiovascular risk.
n6:n3 Ratio. Ecologic studies and limited animal-experimental data suggest that linoleic acid (18:2n-6) may counteract potential benefits of n-3 fatty acids,192-193 but this hypothesis has not been supported by clinical trials or prospective studies in humans.16, 194 A much greater change in the dietary ratio of n-6 fatty acids to n-3 fatty acids can be practically achieved by increasing intake of n-3s (eg, going from no intake of oily fish to 1 serving/wk) compared with lowering intake of n-6s (which are widely consumed in cooking oils, salad dressings, and prepared foods). Thus, for most populations, attention to relative intakes of n-6 vs n-3 fatty acids may be less important than simply increasing n-3 intake.
Aquaculture. Concerns exist about sustainability of some aquaculture and commercial fishing practices.195-197 Conversely, aquaculture contributes to global fish production,198 and sustainability concerns are not unique to aquaculture or fishing but also exist for agricultural, forestry, freshwater, atmospheric, and energy resources.195, 199-200 Some progress has been made, such as changes in fish feeds to reduce dependence on fish meal or oil.195 Given the importance of n-3 PUFAs for health, balance must be achieved between environmental and economic concerns to allow sustainable, financially viable aquaculture and commercial fishing.195-196,199
Plant Sources. Alpha-linolenic acid (ALA) (18:3n-3) is an n-3 fatty acid present in flaxseed, canola, soybeans, and walnuts.121 In humans, ALA is converted to EPA in small quantities (in women more than men); further conversion to DHA is very limited.201 Consumption of ALA (eg, 2-3 g/d) may reduce cardiovascular risk202 or affect neurodevelopment, but benefits are less established compared with those for EPA and DHA.
|
|
| |
| |
|
|
|