| |
Effect of Pioglitazone Compared With Glimepiride on Carotid Intima-Media Thickness in Type 2 Diabetes
|
| |
| |
JAMA, Early Release Article, posted November 13, 2006
A Randomized Trial
Theodore Mazzone, MD; Peter M. Meyer, PhD; Steven B. Feinstein, MD; Michael H. Davidson, MD; George T. Kondos, MD; Ralph B. DfAgostino, Sr, PhD; Alfonso Perez, MD; Jean-Claude Provost, MD; Steven M. Haffner, MD
Department of Medicine, Section of Endocrinology, Diabetes and Metabolism (Dr Mazzone) and Section of Cardiology (Dr Kondos), University of Illinois College of Medicine, Chicago; Departments of Preventive Medicine (Dr Meyer) and Medicine, Section of Cardiology (Drs Feinstein and Davidson), Rush University Medical Center, Chicago; Department of Mathematics, Statistics and Consulting Unit, Boston University, Boston, Mass (Dr DfAgostino); Takeda Global Research and Development Inc, Lincolnshire, Ill (Dr Perez); Synarc, Paris, France (Dr Provost); and Department of Medicine, University of Texas Health Science Center at San Antonio (Dr Haffner).
JAMA. 2006;296:(doi:10.1001/jama.296.21.joc60158).
"....In this randomized trial of 462 patients with type 2 DM (diabetes0, we found that, compared with glimepiride, pioglitazone reduced Carotid artery intima-media thickness (CIMT) progression, a validated surrogate end point for coronary artery disease and cardiovascular risk. The analysis used automated digital edge-detection technology and included multiple measurements in each carotid artery segment.... Our results demonstrate reduction of CIMT progression with pioglitazone treatment in a cohort with a better level of management of cardiovascular risk factors (ie, a higher rate of statin use, LDL-C levels near 100 mg/dL [2.6 mmol/L], and near-optimal blood pressure control) compared with previously reported cohorts.... In patients with type 2 DM, measurement of CIMT significantly improves prediction of cardiovascular disease risk compared with the Framingham Risk Score. It has also been shown that changes in CIMT over time correlate with future cardiovascular event rates.18-19 Diabetes, which confers increased risk of cardiovascular disease, also accelerates CIMT progression... Thiazolidinedione treatment can also positively modify blood pressure, glycemia, and lipid levels... Additional data will be needed to determine the clinical significance of these findings; specifically, whether a strategy of routine use of pioglitazone instead of glimepiride substantially reduces major cardiovascular events."
ABSTRACT
Context Carotid artery intima-media thickness (CIMT) is a marker of coronary atherosclerosis and independently predicts cardiovascular events, which are increased in type 2 diabetes mellitus (DM). While studies of relatively short duration have suggested that thiazolidinediones such as pioglitazone might reduce progression of CIMT in persons with diabetes, the results of longer studies have been less clear.
Objective To evaluate the effect of pioglitazone vs glimepiride on changes in CIMT of the common carotid artery in patients with type 2 DM.
Design, Setting, and Participants Randomized, double-blind, comparator-controlled, multicenter trial in patients with type 2 DM conducted at 28 clinical sites in the multiracial/ethnic Chicago metropolitan area between October 2003 and May 2006. The treatment period was 72 weeks (1-week follow-up). CIMT images were captured by a single ultrasonographer at 1 center and read by a single treatment-blinded reader using automated edge-detection technology. Participants were 462 adults (mean age, 60 [SD, 8.1] years; mean body mass index, 32 [SD, 5.1]) with type 2 DM (mean duration, 7.7 [SD, 7.2] years; mean glycosylated hemoglobin [HbA1c] value, 7.4% [SD, 1.0%]), either newly diagnosed or currently treated with diet and exercise, sulfonylurea, metformin, insulin, or a combination thereof.
Interventions Pioglitazone hydrochloride (15-45 mg/d) or glimepiride (1-4 mg/d) as an active comparator.
Main Outcome Measure Absolute change from baseline to final visit in mean posterior-wall CIMT of the left and right common carotid arteries.
Results
Mean change in CIMT was less with pioglitazone vs glimepiride at all time points (weeks 24, 48, 72).
At week 72, the primary end point of progression of mean CIMT was less with pioglitazone vs glimepiride (-0.001 mm vs +0.012 mm, respectively; difference, -0.013 mm; 95% confidence interval, -0.024 to -0.002; P = .02).
Pioglitazone also slowed progression of maximum CIMT compared with glimepiride (0.002 mm vs 0.026 mm, respectively, at 72 weeks; difference, -0.024 mm; 95% confidence interval, -0.042 to -0.006; P = .008).
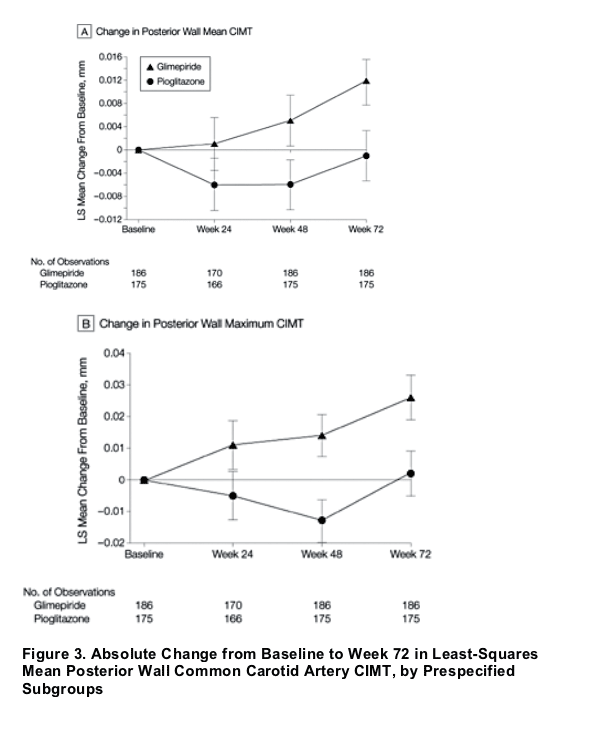
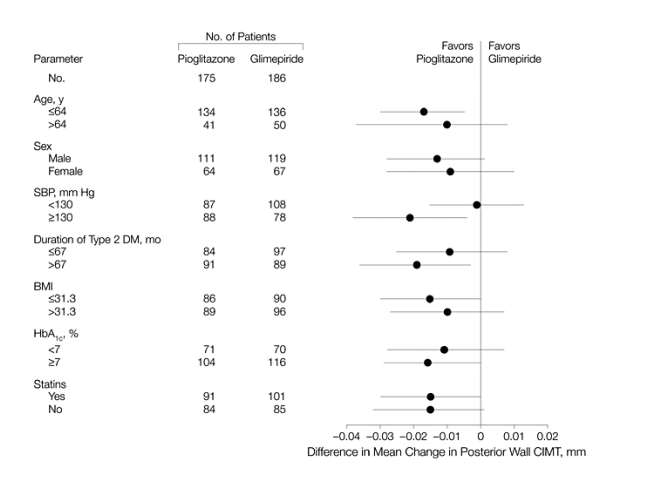
The beneficial effect of pioglitazone on mean CIMT was similar across prespecified subgroups based on age, sex, systolic blood pressure, duration of DM, body mass index, HbA1c value, and statin use.
-- At the end of the study, triglycerides levels decreased 13.5% in the pioglitazone group and increased 2.1% in the glimepiride group.
-- In the pioglitazone group, HbA1c values decreased by week 16 and remained relatively stable throughout the study. There was a rapid decrease in HbA1c values in the glimepiride group, followed by a gradual increase by week 72. There was no significant difference in the HbA1c values between the 2 treatment groups until week 48.
--For HDL-C levels, there was a significant increase with pioglitazone compared with glimepiride treatment at week 24; this increase was maintained throughout follow-up.
--At the end of the study, LDL-C levels increased 5.8% and 1% in the pioglitazone and glimepiride groups, respectively (P = .12)
-- Overall, few patients had a clinical event. No cardiovascular deaths were reported, and there was 1 noncardiovascular death from pancreatic carcinoma in an 80-year-old woman. A numerically higher incidence for clinical end points was observed in the glimepiride compared with the pioglitazone group, and coronary revascularization contributed most to this
-- Peripheral edema was more common with pioglitazone than with glimepiride. Treatment-limiting edema occurred in 4 pioglitazone-treated participants. On average, weight gain was more frequent with pioglitazone than with glimepiride throughout the study but was rarely treatment-limiting. One participant in each group discontinued treatment due to weight gain. At the final visit, mean weight gain was 3.2 (SD, 5.4) kg for pioglitazone and 1.0 (SD, 3.7) kg for glimepiride (P<.001).
Conclusion
Over an 18-month treatment period in patients with type 2 DM, pioglitazone slowed progression of CIMT compared with glimepiride.
Figure 2. Change From Baseline to Week 72 in Mean and Maximum CIMT of the Common Carotid Artery
INTRODUCTION
Patients with type 2 diabetes mellitus (DM) have a marked increase in the risk of myocardial infarction (MI), and a substantially worse prognosis after MI compared with patients without diabetes.1-3 In recent years, it has become apparent that optimal control of blood pressure and low-density lipoprotein cholesterol (LDL-C) level can substantially reduce excess cardiovascular risk in patients with diabetes.4-6 However, even with optimal control of these potent cardiovascular risk factors, incremental risk for cardiovascular events remains high compared with individuals without diabetes.2-3,6 New approaches are, therefore, needed to further reduce cardiovascular risk in patients with diabetes.
Emerging evidence suggests that thiazolidinediones could be useful for reducing cardiovascular risk. In isolated vessel-wall cells, troglitazone, pioglitazone, and rosiglitazone have been shown to modulate gene expression in a manner that would be predicted to be atheroprotective in vivo.7-8 In humans, these agents have been shown to have beneficial effects on systemic inflammatory and coagulation markers, lipoprotein profile, and endothelial cell function.9-12 Some of these beneficial effects may be independent of effects on glycemia.13-15 In animal models of atherosclerosis, thiazolidinediones have been shown to reduce atherosclerotic plaque area independent of changes in glycemia or lipid profile.16-17
When investigating the usefulness of therapies for preventing cardiovascular events, several surrogate end points for estimating future risk of such events have been evaluated. The measurement of carotid intima-media thickness (CIMT) is among the best validated of these surrogate end points.18 CIMT has been shown to highly correlate with risk of future cardiovascular events, and changes in CIMT over time have additional predictive value.18-19 Statins, established agents for reducing risk of cardiovascular disease events, have been shown to reduce progression of CIMT.18, 20
There have been recent reports examining the effect of thiazolidinediones on CIMT in diabetes.13, 21-24 Minamikawa et al23 reported that troglitazone compared with no added treatment reduced CIMT at 3 and 6 months in 135 Japanese patients. Langenfeld et al22 compared pioglitazone with glimepiride in 173 white German participants and reported a reduction in CIMT at 24 weeks. The participants had a baseline systolic blood pressure of approximately 148 mm Hg and an LDL-C level of approximately 136 mg/dL (3.5 mmol/L). In spite of this elevated LDL-C level, statin use was less than 20% at the start of the study. Hodis et al24 recently reported results from 299 patients with type 2 DM. This cohort was more than 66% female and more than 86% Hispanic American and was randomized to receive troglitazone or placebo for 2 years. Overall, the change in CIMT was not different between the 2 treatment groups, although a beneficial effect of troglitazone was observed in the subgroup with a baseline CIMT of 0.8 mm or greater. Because of important issues related to small cohort size, short duration of treatment, homogeneity of study population with respect to race/ethnicity, the presence of uncontrolled cardiovascular risk factors, and inconsistent results, there remains an important question regarding the effect of thiazolidinediones on CIMT in type 2 DM.
In this article, we report the findings of a long-term randomized and comparator-controlled clinical trial conducted in patients with type 2 DM recruited from an ethnically/racially diverse population of a large US metropolitan area. We compared the effect of pioglitazone with that of glimepiride on progression of CIMT. Glimepiride was chosen as a comparator because a placebo control could not be ethically justified in terms of maintaining adequate glycemic control. In addition, glimepiride represents a class of drugs that is commonly used to treat diabetes in the United States, and its mechanism of action is distinct from that of pioglitazone.
METHODS
Study Design and Participants
The CHICAGO (Carotid Intima-Media Thickness in Atherosclerosis Using Pioglitazone) trial was a prospective, randomized, double-blind, comparator-controlled, multicenter study conducted between October 2003 and May 2006 in a multiracial and multiethnic population at 28 clinical sites in the Chicago, Ill, metropolitan area. Individuals eligible for participation were men and women between the ages of 45 and 85 years with type 2 DM by American Diabetes Association criteria25 who were newly diagnosed with type 2 DM that was diet-controlled or treated with sulfonylurea or metformin monotherapy, sulfonylurea/metformin combination therapy, or any of these plus insulin. Individuals taking medication for glycemia were included if they had glycosylated hemoglobin (HbA1c) values of 6.5% or greater and less than 9%; those not taking medication for glycemia were included if they had HbA1c values of greater than 6.5% and less than 10%.
Exclusion criteria included symptomatic coronary artery disease, cerebrovascular disease, or peripheral artery disease; functional New York Heart Association class III or IV heart failure; left ventricular dysfunction measured as left ventricular ejection fraction less than 40%; current use of diuretics or angiotensin-converting enzyme inhibitors for the treatment of heart failure; or significant cardiac valvular disease. Individuals also were excluded if they had been treated with a thiazolidinedione within 12 weeks of treatment randomization; did not respond to or were intolerant of sulfonylurea or thiazolidinedione treatment; required more than 2 oral agents for glycemic control; had unexplained microscopic hematuria, a triglycerides level greater than 500 mg/dL (5.7 mmol/L), elevated serum creatinine level, decreased hemoglobin level, an alanine transaminase level of 2.5 or more times the upper limit of normal; had active liver disease or jaundice; or weighed more than 135 kg or had a body mass index (calculated as weight in kilograms divided by height in meters squared) greater than 45.
Race/ethnicity was initially divided by self-identification as white, black, Hispanic, Oriental, Native American, or other. All of the participants in the "other" category were Asian and so were pooled into an Oriental/Asian group. Race/ethnicity was monitored to allow estimation at the end of the study if the cohort generally reflected the racial/ethnic makeup of patients with type 2 DM in the United States. The study complied with the International Conference for Harmonisation-Good Clinical Practice guidelines, the World Medical Association Declaration of Helsinki, and local regulations. The study protocol was approved by central or local institutional review board committees, and all participants provided written informed consent.
Eligible participants received randomized treatment with pioglitazone hydrochloride (15-45 mg/d) or glimepiride (1-4 mg/d). The initial study drug dose was based on sulfonylurea use and dose at study entry. Patients not taking sulfonylurea or taking a low dose of sulfonylurea started taking daily pioglitazone (15 mg) or glimepiride (1 mg). All other patients were given daily pioglitazone (30 mg) or glimepiride (2 mg). Study drug doses were titrated to reach and maintain target glycemic goals defined as a fasting plasma glucose level of 140 mg/dL (7.8 mmol/L) or lower. The use of metformin or insulin was allowed in either group to reach glycemic goals.
The study protocol specified following American Diabetes Association guidelines for lipid and blood pressure control that were current at the start of the study.26-27 Participants received randomized study drug (pioglitazone or glimepiride) within 3 weeks of screening. For participants requiring the addition or adjustment of statin dose to meet American Diabetes Association targets, an additional time for statin dose stabilization prior to randomization (up to 7 weeks) was permitted. Study visits were scheduled at 4, 8, 16, 24, 32, 40, 48, 60, and 72 weeks after the randomization visit. CIMT was evaluated at baseline and at 24, 48, and 72 weeks (or at the time of early termination). Fasting plasma glucose levels were determined at all visits, HbA1c values at all visits after week 8, and lipid levels (triglycerides, LDL-C, high-density lipoprotein cholesterol [HDL-C], and total cholesterol) at weeks 24, 48, and 72. Adverse events were reported at each study visit. Study drug adherence was assessed at each study visit by pill count and calculated as percentage of pills taken. The adherence rates were 94.9% and 95.5% for pioglitazone and glimepiride, respectively.
The primary end point of the study was absolute change from baseline to final visit in mean posterior-wall CIMT in the right and left common carotid arteries. Absolute change in maximal CIMT from baseline to final visit was included as a secondary end point. Prespecified subgroups for analysis included age, sex, systolic blood pressure, duration of diabetes, body mass index, HbA1c value, and use of statins.
There were 2 composite clinical end points. One composite end point included cardiovascular mortality, nonfatal MI, or nonfatal stroke. The other composite end point included these plus coronary revascularization, carotid endarterectomy/carotid stenting, hospitalization for unstable angina, or hospitalization for congestive heart failure. A clinical end point committee adjudicated events contributing to the composite clinical end points in a blinded fashion. There were no interim data analyses, but a data and safety monitoring board reviewed all safety information every 6 weeks.
Laboratory Measurements
The following analyses were performed by Clinical Reference Laboratory, Lenexa, Kan: levels of triglycerides, total cholesterol, and plasma glucose in blood samples using standard enzymatic methods (Roche Diagnostics, Indianapolis, Ind); levels of HDL-C and LDL-C by direct methods (Roche); and HbA1c values by high-performance liquid chromatography (Bio-Rad, Hercules, Calif).
Measurement of Carotid Intima-Media Thickness
Carotid arteries were imaged by high-resolution B-mode carotid artery ultrasound using an HDI 5000 ultrasound system with a linear-array 7.5-MHz transducer (Phillips Medical Systems NA, Bothell, Wash). All scanning throughout the study was performed by a single ultrasonographer at the same location using the same equipment. Sonographer performance was evaluated by determining the difference between 2 complete examinations of 30 participants performed at least 1 week apart. The mean difference between the 2 readings was 0.002 (SD, 0.058) mm.
The ultrasound beam was adjusted to obtain longitudinal scans of the right and left common carotid arteries to clearly visualize 2 parallel echogenic lines corresponding to the blood-intima and media-adventitia interface on the posterior wall of the artery. Once the imaging position and gain were selected, a sequence of images was digitally recorded using end-diastolic electrocardiographic gating, ie, recording for at least 1 second along with the electrocardiogram tracing to allow for end-diastolic image identification. After the baseline ultrasound examination, masking software (Io-Mask, Synarc, Paris, France) was used to match follow-up scans to optimize alignment to the baseline scan.
Databasing, quality control, and prereading were performed by a central imaging laboratory (Synarc). The digital images were electronically transmitted to Synarc, where the highest-quality end-diastolic images were selected by a trained technician. All measurements were performed in the same artery region throughout the study. Images were blinded according to visit and treatment group and forwarded to a single blinded expert reviewer (S.B.F.). A non-gain-dependent software program (Io-QIMT, Synarc-IoDP Medical Imaging Research) was used to analyze the images and calculate the CIMT using automated edge detection to locate the lumen-intima and media-adventitia echo boundaries at subpixel resolution, as previously described.28-29 The expert reader performance was evaluated as described for the sonographer. The mean difference between replicate readings was 0.007 (SD, 0.046) mm.The CIMT was averaged over 70 to 100 individual measurements taken along a 1-cm segment of the common carotid artery proximal to the bifurcation.
Statistical Analysis
Sample size was calculated based on the assumption of a 0.08-mm group difference in the primary end point at the end of the study, with an SD of 0.224 for individual differences and 90% power for a 2-sided, 2-sample t test at a .05 significance level. Sample size was set to 200 per group in anticipation of a 20% dropout rate. Early in the recruitment the steering committee decided to include all patients who were already in the screening process when the 400 total was reached, to adjust for a somewhat higher than expected early dropout rate.
Unless otherwise noted, analyses were based on intention-to-treat and last-observation-carried-forward principles. Three sites had fewer than 6 participants, and each was pooled with a neighboring site when adjusting for site differences. All treated participants were included who had baseline observations and follow-up observations within 14 days after the last dose of study drug for CIMT measures and 7 days after last dose for other measures. Serious adverse events were included if they occurred within 30 days of the last dose of study medication.
Descriptive statistics were used to characterize participants at baseline by treatment group. Medications used within 8 weeks prior to screening are also reported, with combination antihypertensive medications (combinations of angiotensin-converting enzyme inhibitors, -blockers, angiotensin II receptor blockers, or diuretics) counted in both categories. Baseline group comparisons were adjusted for site, using 2-way analysis of variance for continuous measures and Cochran-Mantel-Haenszel tests for categorical measures.
For primary and secondary end points, analysis of covariance analyses were used and included adjustment for fixed effects of site and CIMT baseline values. The same structure was used for models for relative changes in HbA1c values and levels of HDL-C. Significance of treatment effects was assessed using F tests. Time course was plotted using the LSMEANS function in SAS to show adjusted mean estimates with standard error bars. All analyses were prespecified in the statistical analysis plan, with significance set at .05 (2-sided). A sensitivity analysis of the primary end point analysis used 10-fold multiple imputation, modeling missing final observations using age, sex, treatment group, pooled site, and baseline CIMT.30 Analyses were performed in R version 2.3.0 (R Foundation for Statistical Computing; available at http://www.R-project.org) and SAS version 8.2 (SAS Institute Inc, Cary, NC).
RESULTS
Of the 1346 patients screened for eligibility, 462 (34%) were randomly assigned to treatment (Figure 1). The study was completed by 68% of the pioglitazone-treated and 72% of the glimepiride-treated patients. The reasons for study discontinuation were generally similar between treatment groups. Within the intention-to-treat population, those patients who had both a baseline and one qualifying postbaseline CIMT image were included in the CIMT analysis (CIMT population). This included 76% and 81% of patients from the pioglitazone and glimepiride groups, respectively. One hundred seventy-five pioglitazone-treated and 186 glimepiride-treated patients met the criteria for inclusion for the CIMT analysis.
The treatment groups were well balanced for baseline demographic and clinical characteristics (Table 1). More participants in the glimepiride group had a history of MI (31 vs 18) and used aspirin or diuretics. The majority of patients entering the study were taking an oral diabetes treatment regimen, and most were receiving treatment for hypertension and lipid abnormalities. Glycemic control was good, with a mean HbA1c value of 7.4%. Blood pressure was well controlled, and a majority of patients were using renin-angiotensin system modulators. The majority of patients were taking statin therapy at baseline, and over the course of the study statin use increased to 57.4% and 60.5% in the pioglitazone and glimepiride groups, respectively. The CIMT population was similar to the intention-to-treat population with respect to baseline demographics and clinical characteristics.
Figure 2A shows the mean change from baseline to week 72 in posterior-wall mean CIMT of the right and left common carotid arteries over time. The baseline mean CIMT was 0.771 (SD, 0.008) mm and 0.779 (SD, 0.008) mm in the pioglitazone and glimepiride groups, respectively. The change from baseline at final visit (the prespecified primary end point) in the pioglitazone group was -0.001 mm; the change in the glimepiride group was +0.012 mm. The difference in absolute change from baseline in the pioglitazone compared with the glimepiride group was -0.013 mm (95% confidence interval [CI], -0.024 to -0.002; P = .02). As an alternative to last observation carried forward, 10-fold multiple imputations were performed for missing values and confirmed a treatment effect of 0.013 mm (95% CI, -0.024 to -0.001; P = .03).
Change from baseline to week 72 in posterior wall maximum CIMT was a secondary end point (Figure 2B). Baseline maximum CIMTs were 1.038 (SD, 0.0101) mm and 1.042 (SD, 0.0101) mm in the pioglitazone and glimepiride groups, respectively. The final maximum CIMT increased by 0.002 mm in the pioglitazone group and by 0.026 mm in the glimepiride group. The treatment-group difference was -0.024 mm (95% CI, -0.042 to -0.006; P = .008). Changes in maximal CIMT over time were similar to those observed for mean CIMT. The small excess of participants with a history of MI in the glimepiride group, when included in a model for the primary end point, was not a significant predictor. The favorable treatment effect for pioglitazone was uniform across all prespecified subgroups for mean CIMT analysis (Figure 3), including statin users and nonusers.
Absolute changes in 2 important metabolic parameters over the course of the study are shown in Figure 4. In the pioglitazone group, HbA1c values decreased by week 16 and remained relatively stable throughout the study. There was a rapid decrease in HbA1c values in the glimepiride group, followed by a gradual increase by week 72. There was no significant difference in the HbA1c values between the 2 treatment groups until week 48. For HDL-C levels, there was a significant increase with pioglitazone compared with glimepiride treatment at week 24; this increase was maintained throughout follow-up.
Mean baseline triglycerides levels were 178.6 (SD, 8.1) mg/dL (2.02 [SD, 0.092] mmol/L) and 170.4 (SD, 8.1) mg/dL (1.93 [SD, 0.092] mmol/L) in the pioglitazone and glimepiride groups, respectively. At the end of the study, triglycerides levels decreased 13.5% in the pioglitazone group and increased 2.1% in the glimepiride group (treatment difference, 15.6%; 95% CI, 24.0% to 7.3%; P<.001). Mean baseline LDL-C levels were 113.8 (SD, 2.4) mg/dL (2.95 [SD, 0.062] mmol/L) in the pioglitazone group and 111.3 (SD, 2.4) mg/dL (2.88 [SD, 0.062] mmol/L) in the glimepiride group. At the end of the study, LDL-C levels increased 5.8% and 1% in the pioglitazone and glimepiride groups, respectively (P = .12). At the end of the study, there was a decrease of 2.0 (SD, 13.8) mm Hg and 0.3 (SD, 16.1) mm Hg in systolic blood pressure in the pioglitazone and glimepiride treatment groups, respectively (P = .27).
Table 2 presents the number of patients with an adjudicated first event in prespecified clinical end points. Overall, few patients had a clinical event. No cardiovascular deaths were reported, and there was 1 noncardiovascular death from pancreatic carcinoma in an 80-year-old woman. A numerically higher incidence for clinical end points was observed in the glimepiride compared with the pioglitazone group, and coronary revascularization contributed most to this. Only 1 adjudicated event (a coronary revascularization in the pioglitazone group) occurred in patients with a history of MI at baseline. Congestive heart failure occurred in 1 pioglitazone-treated patient. A single second event (a coronary revascularization performed 1 year after the initial event) occurred in a patient randomized to receive glimepiride.
Table 3 shows the reporting rates for all adverse events. The frequency and type of adverse events seen in the CHICAGO trial are consistent with those seen in previous studies.31 As expected, hypoglycemia was slightly more common with glimepiride than with pioglitazone and resulted in termination of 2 participants in the glimepiride group and 1 in the pioglitazone group. Peripheral edema was more common with pioglitazone than with glimepiride. Treatment-limiting edema occurred in 4 pioglitazone-treated participants. On average, weight gain was more frequent with pioglitazone than with glimepiride throughout the study but was rarely treatment-limiting. One participant in each group discontinued treatment due to weight gain. At the final visit, mean weight gain was 3.2 (SD, 5.4) kg for pioglitazone and 1.0 (SD, 3.7) kg for glimepiride (P<.001).
COMMENT
In this randomized trial of 462 patients with type 2 DM, we found that, compared with glimepiride, pioglitazone reduced CIMT progression, a validated surrogate end point for coronary artery disease and cardiovascular risk.18-19 The CHICAGO trial was conducted in a single geographic region, allowing measurement of CIMT to be performed at a single location by a single sonographer. The analysis used automated digital edge-detection technology and included multiple measurements in each carotid artery segment. Our study population was recruited from a racially and ethnically diverse population of a large US city and generally reflects the diversity of the type 2 DM population in the United States. Our results demonstrate reduction of CIMT progression with pioglitazone treatment in a cohort with a better level of management of cardiovascular risk factors (ie, a higher rate of statin use, LDL-C levels near 100 mg/dL [2.6 mmol/L], and near-optimal blood pressure control) compared with previously reported cohorts.22 Our study also included repeated measurements up to 72 weeks, beyond the 3- to 6-month treatment period in previous trials using pioglitazone.13, 21-22 A prespecified subgroup analysis based on age, sex, systolic blood pressure, duration of type 2 DM, body mass index, HbA1c value, and statin use showed a uniform beneficial effect of pioglitazone treatment.
Systolic blood pressure was reduced by a small amount in both treatment groups. This effect was slightly larger in the pioglitazone group, but treatment differences did not reach statistical significance. Prespecified cardiovascular end points were adjudicated in a double-blinded fashion by an independent panel and more of these events, mostly related to coronary revascularization, occurred in the glimepiride group (Table 2). There was 1 case of new congestive heart failure with pioglitazone therapy, while hypoglycemia was slightly more common with glimepiride. As expected, edema and weight gain were more common in the pioglitazone group. CIMT has been extensively evaluated as a surrogate marker of atherosclerosis and cardiovascular risk.18-19 It has recently been suggested that changes in maximum CIMT may be preferred for measuring treatment-related changes in carotid atherosclerosis.32 Our results demonstrate beneficial effects of pioglitazone on progression for both mean and maximal CIMT values.
In patients with type 2 DM, measurement of CIMT significantly improves prediction of cardiovascular disease risk compared with the Framingham Risk Score.33 It has also been shown that changes in CIMT over time correlate with future cardiovascular event rates.18-19 Diabetes, which confers increased risk of cardiovascular disease, also accelerates CIMT progression.34 However, CIMT progression rates vary widely in patients with diabetes, from 0.083 mm over 6 months to 0.007 mm over 1 year.34-35 In a recent review of 11 CIMT intervention trials in diabetes,36 the mean CIMT progression rate in the control groups was 0.034 mm per year but varied considerably between trials based on level of control of cardiovascular risk factors. The low rate of progession in the control group of the current study likely reflects the control of systolic blood pressure and LDL-C level.
The beneficial effect of pioglitazone on CIMT in patients with type 2 DM is consistent with results of the recently reported PROactive (Prospective Pioglitazone Clinical Trial in Macrovascular Events) study.37 This trial randomized more than 5000 patients with type 2 DM who had evidence of macrovascular disease to receive pioglitazone or placebo in addition to existing therapy. While treatment with pioglitazone did not significantly reduce the risk of the composite primary end point (which included death from any cause, nonfatal MI, stroke, acute coronary syndrome, leg amputation, coronary revascularization, or leg revascularization), it did significantly reduce by 16% the risk of the main secondary end point (a composite of all-cause mortality, MI, or stroke).
Several potential mechanisms can be considered for a beneficial effect of pioglitazone on atherosclerosis. Treatment with thiazolidinediones has been shown to modify nontraditional markers of cardiac risk such as circulating inflammatory and coagulation markers and to improve endothelial-cell function.9-12,38-39 Thiazolidinedione treatment can also positively modify blood pressure, glycemia, and lipid levels.9-12 In the current study, blood pressure changes were not significantly different between the pioglitazone and glimepiride groups. HbA1c values were reduced more in the pioglitazone group compared with the glimepiride group (by 0.32% at 72 weeks). However, it is noteworthy that the treatment advantage for pioglitazone on HbA1c values in this study did not become significant until week 48. In prior studies, treatment with pioglitazone has been shown to have a substantial benefit on diabetic dyslipidemia, including increasing HDL-C levels and reducing triglycerides levels.12 Both of these effects were observed in our study and could have contributed to improvement in CIMT. Finally, it also remains possible that thiazolidinediones can have a directly beneficial effect on the vessel wall.16-17
Our study has several limitations. First, it was not powered to detect a difference in cardiovascular end points and, therefore, does not establish that treatment with pioglitazone compared with glimepiride will reduce these end points in patients with type 2 DM. Because we used glimepiride as an active comparator, we also cannot definitively rule out that the treatment difference was due to a proatherogenic effect of glimepiride. We believe, however, that this explanation is somewhat unlikely in view of the fact that the treatment difference was largely the result of an effect of pioglitazone to suppress or delay the progression of CIMT. Our study also had a dropout rate that approximated 30%. However, dropout rates were balanced in the 2 treatment groups, and the participants who remained in the study (ie, the CIMT population) were similar to those in the intention-to-treat population (Table 1). In addition, an analysis of baseline characteristics of participants who dropped out showed no difference compared with those who remained in the study. Finally, thiazolidinediones may cause acute changes in intravascular volume and affect vascular tone. Such changes also result from antihypertensive therapy, and an issue has been discussed in the literature regarding a potential role for changes in intravascular volume, vascular tone, or both in producing rapid changes in CIMT.18 In the current study, the observation that treatment difference appeared to increase over time argues against an important role for changes in intravascular volume or vascular tone. In a recent evaluation of the effect of antihypertensive therapy on CIMT, Zanchetti et al40 concluded that only 1% of CIMT change could be attributed to overall change in carotid artery diameter.
Notwithstanding these limitations, our results demonstrate, in a relatively large and long-term randomized trial, that pioglitazone slowed progression of CIMT compared with glimepiride. This benefit was measured in participants with excellent blood pressure control, statin use greater than 50%, and mean LDL-C levels of 113.8 (SD, 2.4) mg/dL (2.95 [SD, 0.062] mmol/L). Additional data will be needed to determine the clinical significance of these findings; specifically, whether a strategy of routine use of pioglitazone instead of glimepiride substantially reduces major cardiovascular events.
|
|
| |
| |
|
|
|