| |
A Prospective Controlled Study of Neurodevelopment in HIV-Uninfected Children Exposed to Combination Antiretroviral Drugs in Pregnancy
|
| |
| |
PEDIATRICS Vol. 118 No. 4 October 2006, pp. e1139-e1145
Ariane Alimenti, MDa, John C. Forbes, BSc, MB, ChB, FRCP(C)a, Tim F. Oberlander, MD, FRCP(C)a, Deborah M. Money, BSc, MD, FRCSCb, Ruth E. Grunau, BA, MA, EdD, PhD/RPsycha, Michael P. Papsdorf, PhDc, Evelyn Maan, RNd, Lesley J. Cole, RN, BSNe and David R. Burdge, BSc, MD, FRCP(C)d
a Departments of Pediatrics
b Obstetrics and Gynecology
d Medicine, University of British Columbia, Vancouver, British Columbia, Canada
c Centre for Community Child Health Research
e Maternal Fetal Medicine, Children's and Women's Health Centre of British Columbia, Vancouver, British Columbia, Canada
ABSTRACT
OBJECTIVE. Our intent was to investigate the neurodevelopment of HIV-uninfected children exposed to combination highly active antiretroviral therapy in pregnancy compared with children not exposed to highly active antiretroviral therapy but with similar socioeconomic backgrounds.
PATIENTS AND METHODS. A prospective controlled cross-sectional study of the neurodevelopment of children exposed to highly active antiretroviral therapy versus those not exposed was performed by using the Bayley Scales of Infant Development and Vineland Adaptive Behavior Scales at 18 to 36 months of age. The highly active antiretroviral therapy-exposed children were born to HIV-infected women but were uninfected themselves. The control children were born to HIV-uninfected women with similar anticipated socioeconomic background (hepatitis C infected and high proportion of substance use). Sociodemographic, clinical, highly active antiretroviral therapy (antenatal, intrapartum, neonatal), and substance-use histories were collected. Results were compared by using analyses of covariance and 2 analysis.
RESULTS. Thirty-nine highly active antiretroviral therapy-exposed and 24 control children were assessed. All mean scores were lower for those in the highly active antiretroviral therapy-exposed group than those in the control group (Bayley Mental Development Index: 85.4 vs 94.3; Bayley Psychomotor Development Index: 93.4 vs 96.6; Vineland mean communication score: 90.1 vs 94.4; Vineland mean daily-living score: 91.2 vs 93.6; Vineland mean socialization score: 97.1 vs 98.4). However, when maternal substance use during pregnancy was controlled for, there were no significant differences between the groups in any domains assessed. Children in both groups exposed to maternal substance use scored significantly lower than children not exposed in all domains except communication skills. It is important to note that there were no differences between the highly active antiretroviral therapy-exposed children with no substance exposure and the control children with no substance exposure in any of the scores.
CONCLUSIONS. HIV- and highly active antiretroviral therapy-exposed HIV-uninfected children had lower development and adaptive behavior scores when compared with children who had not been exposed. However, these differences were not significant after correcting for maternal substance use, which had a greater impact on neurodevelopment than highly active antiretroviral therapy exposure. These results suggest that perinatal highly active antiretroviral therapy exposure is not associated with altered development and behavior at 18 to 36 months of age.
Highly active antiretroviral therapy (HAART) during pregnancy, intravenous zidovudine at delivery, and oral zidovudine to the neonate dramatically reduce mother-to-child transmission of HIV-1, in the absence of breastfeeding, to <2%.1,2 Although HAART in pregnancy significantly reduces the risk of vertical HIV transmission, concerns exist regarding the potential impact of antiretroviral drug exposure on these children. Most antiretroviral medications cross the placenta, and the potential risk of drug exposure exists throughout the pregnancy. Of particular concern is that nucleoside analogues are known to inhibit mitochondrial DNA -polymerase, thereby altering mitochondrial replication and inducing mitochondrial dysfunction.3,4 Several studies have been published that demonstrate evidence of transient or prolonged mitochondrial compromise in children born to mothers receiving antiretroviral drugs during pregnancy.5,6 However, the long-term relevance of these findings remains unknown.
Studies to date, mostly evaluating monotherapy regimens, have been reassuring regarding the effects of antiretroviral drugs during pregnancy. Long-term follow-up of children exposed perinatally to zidovudine monotherapy in the Pediatric AIDS Clinical Trial Group 219/076 study has not indicated differences in growth, immune function, or neurologic development up to the median age of 4.2 years (range: 3.2-5.6 years).7 In a European multivariate analysis there was an association between prematurity and exposure to combination antiretroviral therapy in pregnancy, with and without a protease inhibitor.8 However, data from a meta-analysis of 7 clinical studies in the United States did not find an association between antiretroviral therapy and preterm delivery, low birth weight (<2500 g), or low Apgar scores.9
Although these studies are reassuring, there are few data available regarding the developmental outcomes of HIV-uninfected children exposed perinatally to antiretroviral therapy. Although organ differentiation occurs mainly during the first trimester of pregnancy, major brain development continues during the second and third trimesters and in the neonatal period; therefore, fetal and neonatal exposure could be a period of increased vulnerability. Indeed, some studies of neurologic effects have raised concerns. In France, 8 uninfected children exposed to zidovudine or zidovudine and lamivudine were reported to have histologic evidence of mitochondrial dysfunction, 5 had neurologic symptoms, and 2 with encephalopathy died.10 In a prospective cohort, uninfected exposed children had a higher risk of neurologic syndromes associated with mitochondrial dysfunction than seen in the general population.11 However, a large retrospective review of 5 cohorts in the United States failed to show clear evidence of clinically relevant mitochondrial diseases in nucleoside-exposed children who died before 5 years of age.12 The European Collaborative Study also showed no evidence of clinical manifestations suggestive of mitochondrial abnormalities in children exposed to antiretroviral therapy in utero.8
Many of the follow-up studies of HIV-uninfected children exposed to antiretroviral therapy have been retrospective studies, and, to date, none of these studies have included a control group of children for direct comparison of neurodevelopmental outcomes. Our study was performed to better address these issues in a prospective fashion.
OBJECTIVES
The primary objective for this study was to determine if HIV-uninfected children with perinatal exposure to HAART display neurodevelopmental abnormalities compared with children from similar socioeconomic backgrounds not exposed to HAART. A secondary objective was to determine if differences in neurodevelopment occur in children exposed to illicit substances of addiction compared with children not exposed to illicit substances.
METHODS
Study Design
We used a cross-sectional design to compare the neurodevelopment of HIV-uninfected children exposed to HAART versus a group of unexposed children with anticipated similar socioeconomic backgrounds. Potential factors of importance to neurodevelopment including maternal history of smoking, alcohol use, and substance use during the pregnancy, obstetric and delivery complications, and family composition, education, and socioeconomic status of the parents were all documented. Children underwent standardized neurodevelopmental assessment at 18 to 36 months of age.
Sample-size calculation indicated that for an expected 10% reduction in neurodevelopment scores and a significance level of P = .05, 30 children were required in each group. Siblings were excluded from the study to assure independence of observations.
Patient Population
Subjects for the HAART-exposed group were selected consecutively from a cohort of HIV-uninfected children born to HIV-positive women who were followed in the provincial tertiary care clinic for HIV-infected women and their children in British Columbia, Canada, from June 2003 to December 2004. All the known HIV-infected pregnant women in British Columbia receive care through this university hospital-associated program. Inclusion criteria for this group of children were: born to HIV-positive mothers, exposed to at least 3 antiretroviral drugs in utero for a minimum of 1 week and to zidovudine during delivery and the neonatal period, HIV-uninfected, and 18 to 36 months of age. We anticipated that a significant proportion of these children would also be exposed to maternal substance use and alcohol. All HIV-uninfected children had at least 2 nonreactive HIV polymerase chain reaction tests between 1 and 6 months of age and had seroreverted on HIV-1 serologic testing. Parents or guardians were informed of the study and offered participation during routine clinic visits. Those who expressed interest were then contacted by telephone by the study coordinator.
The control group was obtained from a cohort of children who were followed in a concurrent province-wide hepatitis C vertical-transmission study headed by one of the co-investigators (D.M.M.). They were born to HIV-negative hepatitis C-infected mothers with a high proportion (note from Jules Levin: don't you think HCV-infected mothers are a poor control group, they might have impaired neuro functioning with or without substance abuse) (49.6%) of injection drug-use history. The control group was chosen in an attempt to match the HAART-exposed group in terms of socioeconomic background and substance use during pregnancy. None of the control children were exposed to antiretroviral therapy. Parents or guardians of control children were contacted by telephone and informed about the study; those who expressed interest were mailed a letter that asked them to contact the study coordinator.
Written informed consent was obtained from all parents/guardians of subjects and controls before study enrollment. The study received approval from the Research Review Committee of Children's and Women's Health Centre of British Columbia and the Clinical Research Ethics Board of University of British Columbia (certificate numbers C01-0135/W01-0036). Children identified as being developmentally delayed were referred to behavioral and developmental programs. For children with more severe effects, psychoeducational assessments will be requested at school age to allow for additional support in the school and home environments.
Neurodevelopmental Measurements
The Bayley Scales of Infant Development-II (BSID-II)13 were used to assess cognitive, language, and psychomotor functioning with the primary goal of identifying developmental delay. The BSID-II is the most widely used scale for assessing development from infancy to 30 months. Results provide Mental Development Index (MDI) and Psychomotor Development Index (PDI) scores, with a mean of 100 and SD of 15.
The Vineland Adaptive Behavior Scales Revised-Survey Form14 was used to assess the areas of communication, daily-living skills, and socialization. This is a well-standardized, norm-based questionnaire based on semistructured interview and has been used extensively to evaluate adaptive skills in young children. Results provide a score with a mean of 100 and SD of 15 for each of the domains.
An experienced, trained examiner administered all the neurodevelopmental assessments and was blinded to cases versus controls. Both neurodevelopmental measurements and a clinic visit including a physical examination by 1 of 2 pediatricians were completed during 1 session lasting <3 hours.
Data Collection and Analysis
Demographic and associated risk-factor data were collected prospectively on predesigned data-collection forms in both study groups. Information was collected from clinical records and through a prospective, detailed, confidential questionnaire administered by the respective study coordinators. Data included type and duration of exposure to HAART during pregnancy; exposure to other substances such as drugs of addiction, alcohol, tobacco, and medications; pregnancy and delivery complications; family composition, highest level of education, and employment and economic status; and highest plasma lactate level recorded between birth and 6 months of age. Serum lactate levels were tested in all HAART-exposed infants at each clinic visit; each infant had a minimum of 4 visits from birth to 6 months of age. Lactate levels were tested as an indirect measure of potential mitochondrial toxicity from perinatal antiretroviral therapy. The technique and results were reported previously.6 An electronic database using Microsoft Access (Redmond, WA) software was used to store all coded study data.
The 2 groups were compared on means by using analysis of covariance to allow for control of specific variables and on frequencies by using 2 tests. In a second step, we also undertook exploratory analyses of factors that could impact neurodevelopment, such as the type and duration of exposure to other substances during pregnancy, the type and duration of exposure to antiretroviral agents, and the timing of exposure (from conception versus third trimester alone). Differences were considered significant for a P value of <.05.
RESULTS
Thirty-nine HAART-exposed subjects and 24 control children were assessed between June 2003 and December 2004. During the study period, of the 64 children born to mothers on HAART, a total of 25 children were unavailable or not eligible: 11 parents/guardians did not return telephone calls, 8 declined participation or did not keep appointments, 3 families had an English-language barrier, and 3 children were excluded because a sibling had participated. One child born with congenital anomalies and developmental delay presumably caused by prolonged fluconazole exposure was excluded from the study. In the control group, of the 54 children eligible, parents of 20 children informed about the study by letter did not participate: 13 did not return telephone calls, and 7 declined participation (5 of which were for reasons related to distance from the study center).
Baseline characteristics of the mothers and children are shown in Table 1. Children in the HAART-exposed group were born at a significantly earlier gestational age (mean: 37.7 vs 39 weeks) and with a lower birth weight (mean: 3028 vs 3410 g) than control-group children. Other baseline characteristics including maternal age at delivery, education level, and employment status did not differ significantly between the 2 groups.
HAART in pregnancy consisted of 2 nucleoside reverse-transcriptase inhibitors for all 39 mothers combined with nevirapine in 22, a protease inhibitor in 13, both nevirapine and a protease inhibitor in 3, and 3 nucleosides in 1. Mean duration of the HAART exposure was 17.7 weeks (range: 1-40 weeks) with a median exposure to zidovudine of 15 weeks (range: 1-39 weeks), lamivudine of 17 weeks (range: 1-39 weeks), nevirapine of 12.5 weeks (range: 1-38 weeks), and Nelfinavir of 16.5 weeks (range: 5-35 weeks). Only 9.4% of the women had <4 weeks antiretroviral therapy during pregnancy. No significant relationships were observed between duration of HAART exposure and the dependent variables except for zidovudine duration, which was positively correlated with the Vineland socialization score (r = 0.42; P = .017).
Mean age at the time of neurodevelopmental assessment, for the HAART-exposed group was 25.9 months (range: 18.1-35.8 months) and for the control group was 22.1 months (range: 17.8-32.8 months). All mean developmental scores on the BSID-II and Vineland scales were lower in the HAART-exposed group than the control group, as shown in Table 2. However, only the BSID-II MDI was significantly different. A greater proportion of HAART-exposed children scored >1 SD below average on the BSID-II MDI (54% vs 25%; P = .025). Among the Vineland domains, differences were observed in the proportion of children scoring >1 SD below average in daily-living skills (33% in HAART-exposed vs 8% in control children; P = .024). Children who scored >2 SD below average in the BSID-II PDI and all Vineland domains were rare in both groups (0-3 per group), and no significant differences were observed between the 2 groups.
Although we anticipated that maternal substance use in pregnancy would be similar in the 2 groups, it was actually disproportionately higher in the HAART-exposed group than in the control group (51% vs12%; P = .002). Maternal injection drug use was 46% in HAART-exposed children and 4% in control children (P < .001). The substances used during pregnancy consisted of cocaine and/or heroin in >90% of the cases. It is noteworthy that 30% of the children in the HAART-exposed group had narcotic withdrawal syndrome, whereas none in the control group were affected (P = .002). Maternal cigarette use was higher in the HAART-exposed group than in the control group (59% vs 33%; P = .028), and alcohol use was not significantly different. Forty percent of the children who were exposed to substance use were also exposed to alcohol during the pregnancy. When maternal substance use during pregnancy was controlled for, none of the differences in neurodevelopment scores between the 2 groups was significant. Children who were exposed to maternal substance use (HAART-exposed and control groups combined) scored lower in all test components than children not exposed (Table 3). These differences reached statistical significance (all P values <.05) for all domains except communication skills.
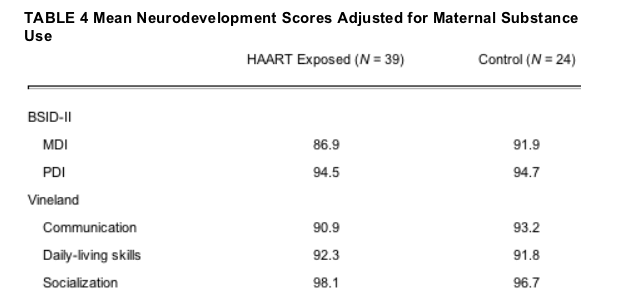
An analysis of covariance was conducted to assess the differences between the 2 study groups controlling for maternal substance use. There were no significant differences between the groups on any of the mean developmental scores on the BSID-II and Vineland scales (all P values >.25). The adjusted group means are presented in Table 4.
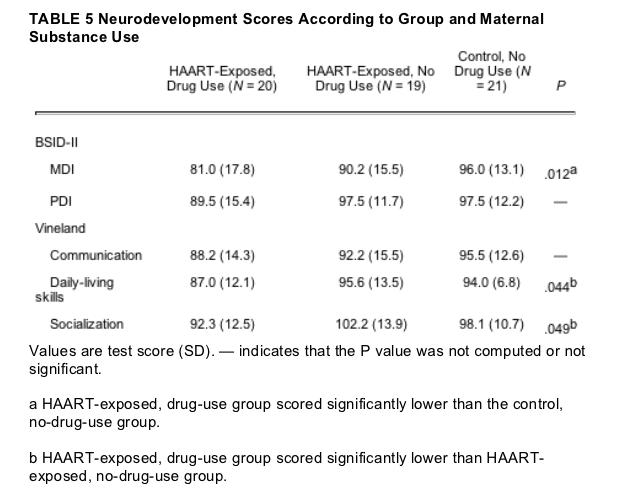
Subgroup analyses were also conducted for the HAART-exposed group, comparing those with maternal substance use to those with no maternal substance use, and to those in the control group with no maternal substance use. It is important to note that there was no difference found between the latter 2 groups. For all outcome measures the means of the control group and the HAART-exposed group with no substance use were closer together than either was to the HAART-exposed group with substance exposure. Differences were noted between the HAART-exposed group with substance use and the control group in the BSID-II MDI result and also with the HAART-exposed group with no substance use in the Vineland daily-living and socialization results. Statistical power is reduced when the group is subdivided. The results of these subgroup analyses are shown in Table 5.
When methadone exposure in the HAART-exposed group was studied independently, significant differences were also observed in BSID-II MDI scores (77.5 in the 13 of 39 who had methadone exposure vs 89.4 in the 26 of 39 who had no methadone exposure; P = .039).
Fourteen children who were born before 37 weeks' gestational age (10 in the HAART-exposed group and 4 in the control group) scored lower on the BSID-II PDI than children who were born at term (87.1 vs 96.4; P = .026), even when controlling for substance use. Similarly, the 9 children who weighed <2500 g at birth (6 in the HAART-exposed group and 3 in the control group) scored significantly lower on the BSID-II PDI and Vineland daily-living skills and socialization domains (85.9 vs 96.2 [P = .036], 84.2 vs 93.4 [P = .032], and 88.4 vs 99.1 [P = .021], respectively) but showed no significant difference in the other domains.
Perinatal complications such as placental abruption, fetal distress, or required resuscitation maneuvers at birth were recorded in 10 (25%) of the HAART-exposed children versus only 1 (4%) of the control children (Table 1). There were too few children with birth complications to carry out statistical comparisons of neurodevelopment scores between those in the exposed and nonexposed groups.
Developmental scores of 9 of 39 children who had hyperlactatemia (>5 mmol/L) at least once during the first 6 months of life did not differ significantly from those with levels that remained at <5 mmol/L at all times during that period.
DISCUSSION
In this prospective cross-sectional study, perinatal HAART-exposed HIV-uninfected children had lower but not dissimilar developmental and behavioral outcomes compared with a HAART-unexposed, HIV-negative, socioeconomically similar control group at 18 to 36 months of age. Moreover, the differences were no longer present when we controlled for maternal substance use in pregnancy. It is important to note that children exposed to maternal substance use in pregnancy had significantly lower development scores than nonexposed children. In subgroup analysis, substance use had a greater impact on neurodevelopment than HAART exposure. Indeed, children exposed to HAART with no maternal substance use had equivalent neurodevelopment scores to control children exposed to neither HAART nor substance use in pregnancy. Overall, we found that maternal substance use was a stronger predictor of a poor neurodevelopmental outcome than was HAART exposure.
Given that HAART regimens involve the use of nucleoside analogues that have the potential to cause mitochondrial toxicity, the issue of potential impact on neurodevelopment is of particular importance. As noted, aside from the children followed in the Pediatric AIDS Clinical Trial Group 219/076 study cohort, all previous investigations of neurodevelopmental outcomes in antiretroviral-drug-exposed patients were retrospective and involved children exposed to either zidovudine alone or dual antiretroviral therapy. This is the first prospective study of neurodevelopment in children exposed to HAART (for a mean of 17.7 weeks exposure in pregnancy to at least 3 antiretroviral drugs) with a control group of non-HAART-exposed children from similar socioeconomic backgrounds. We therefore believe that this study provides additional reassurance of the relative safety of HAART interventions in pregnancy.
An unforeseen limitation in our study was that despite attempting to have a control group well matched from both socioeconomic-background and substance-use perspectives, active maternal substance/alcohol use was present in 51% of the HAART-exposed children but only 12% of the control children. We had anticipated similar rates of exposure to substance use in pregnancy given that the proportion of women with any history of substance use was very similar (61.5% in HAART-exposed and 49.6% in controls). The larger-than-expected difference in active substance use in pregnancy between groups is likely a reflection of the recruitment process, with women in recovery from their substance use in the control group being more likely to volunteer to participate in the study. The control group was from a large provincial study, and those who agreed to be enrolled in the current study were able to devote the time to the study and were keen to participate to receive the neurodevelopmental results for their infants.
Other differences between the groups in our study included earlier gestational age, lower birth weight, and more perinatal complications in the HAART-exposed children. In our study, infants from both groups born before 37 weeks' gestation and with birth weight <2500 g showed lower neurodevelopment scores, even when controlling for maternal substance use. We believe that the lower developmental scores of these children are likely an effect of prematurity and low birth weight rather than a direct consequence of HAART exposure, although this study was not designed to examine effects of prematurity and low birth weight. Maternal substance use has been shown to be associated with lower gestational age and birth weight in HIV-uninfected infants.15
The development and behavioral impact of prenatal exposure to cocaine has been a focus of substantial research; however, the impact of such exposure is highly associated with multiple other risk factors such as prenatal exposure to tobacco, marijuana, alcohol, and the quality of the environment in which the child lives.16 In our study, maternal prenatal substance use contributed to lower development scores, highlighting the importance of accounting for prenatal substance exposures other than HAART on infant development. Substance exposure in this context may also be an indirect measure of other factors influencing neurodevelopment, such as home and family environment and caregiver's health. Home environment during the first years of life plays an important role in a child's neurodevelopment, and the positive effect of intervention programs has been demonstrated.17 Almost one third of the children in this study experienced a change in the family's status (such as placement in foster care or separation from the father) in the preceding year. Data obtained on family composition, parental level of education, income status, and ethnic background were difficult to interpret given the size of the cohort.
Other limitations in our study need mentioning. A number of key maternal pregnancy-related variables could only be estimated, and although we attempted to control for other prenatal drug exposure, we were not able to precisely document the timing, quantity, and duration of prenatal drug and alcohol exposure. As a result, it was impossible to deduce the precise influence of prenatal cocaine and other substance use on developmental outcomes. In addition, our cross-sectional design was not able to determine developmental trajectories. Studying other points in time and using more precise measures of development and behavior (attention, memory, cognition, etc) may be needed to assess the effects of HAART on the developing child. Finally, although a number of significant effects were observed, the sample size of 24 in the control group rendered the study less powerful than expected.
CONCLUSIONS
Although HAART exposure may lead to mitochondrial toxicity, these preliminary results suggest that HAART exposure in HIV-negative children is not associated with altered global measures of development and behavior at 2 years of age. However, our findings do illustrate the importance of accounting for prenatal substance use, which frequently accompanies prenatal HIV and HAART exposure.
There potentially will be millions of HIV-infected women treated with HAART during pregnancy over the next few years, and it is imperative that more information be gained regarding the safety of these therapies in pregnancy and their impact on the exposed uninfected child. An improved understanding of potential toxicities will enable and inform improved approaches to antiretroviral therapies in pregnancy and the neonate. Additional study of developmental outcomes after prenatal HAART exposure needs replication using prospective longitudinal cohort designs that account for multiple substance exposures and social and maternal factors.
|
|
| |
| |
|
|
|