| |
Lipoatrophy Improves After Switch to Abacavir or Tenofovir RAVE Study
|
| |
| |
A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy
AIDS: Volume 20(16) 24 October 2006 p 2043-2050
Moyle, Graeme Ja; Sabin, Caroline Ab; Cartledge, Jonathanc; Johnson, Margaretb; Wilkins, Edmundh; Churchill, Duncani; Hay, Philipd; Fakoya, Adee; Murphy, Mauricef; Scullard, Georgeg; Leen, Cliffordj; Reilly, Geraldinek; for the RAVE (Randomized Abacavir versus Viread Evaluation) group UK
From the aChelsea and Westminster Hospital, London
bRoyal Free & UC Medical School, London
cCamden Primary Care Trust, London
dSt Georges Hospital, London
eNewham General Hospital, London
fBarts and the London NHS Trust, London
gSt Mary's Hospital, London
hNorth Manchester Hospital, Manchester
iBrighton and Sussex University Hospital, Brighton
jWestern General Hospital, Edinburgh
kGilead Sciences, Cambridge, UK.
"....In summary, switching from a thymidine analogue to either tenofovir DF or abacavir leads to statistically significant improvement in limb fat mass over 48 weeks. Both drugs are generally well tolerated and virologically effective. Peripheral lipoatrophy, when clinically apparent, resolves slowly following treatment switching. Where feasible, consideration should be given to proactive switching away from thymidine analogue to either tenofovir DF or abacavir...."
Abstract
Background: Long-term antiretroviral therapy, while dramatically reducing HIV-related morbidity and mortality, is associated with metabolic and morphological changes. Peripheral fat loss, lipoatrophy, appears most associated with prolonged therapy with thymidine nucleoside analogues.
Methods: A randomized, open-label, comparative study of switching from a thymidine nucleoside analogue to either tenofovir disoproxil fumarate (DF) or abacavir in 105 individuals on successful antiretroviral therapy with clinically evident moderate to severe lipoatrophy.
Results:
Individuals were randomized to tenofovir DF (52) or abacavir (53). The switch was well tolerated and the majority of patients completed 48 weeks of study. One individual in the tenofovir DF group and three in the abacavir group discontinued due to drug-related adverse events.
Both groups similarly maintained virological control. Limb fat mass increased similarly in both groups: mean increases by week 48 of 329 and 483 g in tenofovir DF and abacavir groups, respectively [mean 95% confidence interval for difference, -154.3 (range -492.8 to 184.3)]. This change from baseline was statistically significant in both groups (tenofovir DF, P = 0.01; abacavir, P = 0.0001).
Mean total cholesterol, low density lipoprotein cholesterol and triglycerides improved modestly with switching to tenofovir DF but were unchanged with abacavir. The changes in these parameters were significantly greater in the tenofovir DF arm relative to abacavir.
Conclusions: Switching from a thymidine nucleoside analogue to either tenofovir DF or abacavir leads to significant improvement in limb fat mass over 48 weeks. Tenofovir DF may have modest advantages over abacavir for changes in lipids. Peripheral lipoatrophy, when clinically apparent, resolves slowly following treatment switching.
Introduction
Treatment of HIV infection with antiretroviral regimens dramatically reduces HIV-associated morbidity and mortality [1]. However, prolonged therapy may be complicated by adverse effects, including the metabolic syndrome (dyslipidaemia, insulin resistance, visceral adiposity) and subcutaneous lipoatrophy [2]: collectively known as lipodystrophy. The morphological changes of lipodystrophy are stigmatizing and may lead to reduced adherence or treatment discontinuation. The metabolic changes may contribute to an increased risk of cardiovascular events [3].
Reports from clinical trials of thymidine nucleoside analogue-based (stavudine or zidovudine) regimens indicate that the prevalence of lipoatrophy in persons receiving therapy for 3 years is 19% or more [4-7]. Coadministration of a thymidine analogue with some protease inhibitors may further accelerate fat loss [6]. Data from studies using thymidine-sparing regimens have reported normal limb fat mass and few instances of clinical lipoatrophy over prolonged follow-up [7,8], suggesting that, in the future, lipoatrophy may occur less frequently on such regimens.
Lipoatrophy is the most difficult manifestation of lipodystrophy to manage [9]. The only approach to its management that has so far demonstrated benefit in randomized controlled trials is switching therapy from a thymidine analogue to abacavir [10-12]. However, this option is not available to everyone owing to hypersensitivity reactions, intolerance or drug resistance. This study, therefore, investigated the potential effects on limb fat of switching a thymidine analogue to tenofovir DF as an alternative to abacavir. Additionally, as studies with tenofovir DF-based regimens have suggested more favourable effects on total cholesterol and triglycerides than thymidine analogues [7,13], changes in lipid and other metabolic parameters were also investigated.
Methods
This phase IV, open-label, multicentre, randomized, 48-week trial compared the substitution of zidovudine or stavudine with either tenofovir DF or abacavir in patients successfully treated with antiretroviral therapy. The study was performed in 10 UK HIV treatment centres. It was approved by UK local ethics committees. Patients provided written informed consent prior to study entry and the study was conducted according to Good Clinical Practice guidelines. The study was funded and sponsored by Gilead Sciences and was monitored by a data and safety monitoring board.
The primary endpoint of the trial was the change in limb fat mass measured by dual X-ray absorptiometry (DEXA) scan from baseline to 48 weeks. Secondary endpoints were changes in limb fat mass from baseline to week 24; changes in total body and trunk fat mass; changes in visceral, subcutaneous and total adipose tissue [measured by single-slice abdominal computed tomographic (CT) scan at L4]; fasting cholesterol [total, high density lipoprotein (HDL) cholesterol and calculated low density lipoprotein (LDL) cholesterol] and triglycerides, insulin, glucose and lactate at 24 and 48 weeks. Additional endpoints included change in bone mineral density by DEXA scan, the incidence of adverse events, virological rebound (two consecutive HIV-1 RNA measurements > 200 copies/ml) and change in CD4 cell count from baseline to 24 and 48 weeks.
Eligible HIV-1-infected subjects were aged 18 years or over, had been stable on current therapy for > 16 weeks and had no prior exposure to tenofovir, abacavir, adefovir or known resistance to these agents (genotype including 65R, 74V, 115F or 69S mutations or three or more thymidine analogue mutations). Individuals who had received previous dual or monotherapy with a nucleoside reverse transcriptase inhibitor were excluded. Individuals with clinically evident moderate to severe lipoatrophy [14] at one or more body/facial sites, currently receiving zidovudine or stavudine and with documented HIV-1 RNA of < 50 copies/ml on two consecutive occasions were recruited. Women of childbearing potential were requested to use an effective method of contraception. Exclusion criteria included active opportunistic disease or wasting syndrome, receipt of chemotherapy for malignancy, an insulin-sensitizing agent, anabolic steroids in the last 16 weeks (other than testosterone at replacement doses), growth hormone in the last 16 weeks or any agent likely to interact with study drugs. Statin therapy was permitted if at a stable dosage and if commenced > 16 weeks before randomization. Individuals with creatinine > 1.25 times the upper limit of normal were excluded.
Eligible subjects stopped zidovudine/stavudine and were randomized to start either tenofovir DF 300 mg once daily or abacavir 300 mg twice daily. They continued the other antiretroviral drugs in their regimen.
The randomization list, generated using a random number generator and stratified by prior exposure to protease inhibitors and whether the patient was currently receiving zidovudine or stavudine, was held by Gilead Sciences (Foster City, California, USA). After recruitment, each patient's anonymized details were faxed to Gilead, who notified the recruiting clinician of the treatment allocation.
Participants were followed at baseline and at weeks 4, 12, 24, 36 and 48 for adverse effects, full blood count, biochemistry, liver and renal function, insulin, triglycerides and lipids. DEXA and CT scans were performed at baseline, week 24 and week 48.
Statistical methods
Changes in quantitative measures from baseline to weeks 24/48 within each treatment group were generally normally distributed so were tested for significance using paired t-tests; comparisons of the changes between treatment groups were performed using unpaired t-tests. Where changes were non-normally distributed, values were either log-transformed or non-parametric tests (Mann-Whitney U tests) were performed. All qualitative variables were analysed using χ2 tests or Fishers exact tests, as appropriate.
Analyses of lipid changes were based on all values as measured, disregarding any information on the use of lipid-lowering therapy. Sensitivity analyses were performed in which those individuals who started lipid-lowering therapy had their 48-week values replaced by the most recent value prior to lipid-lowering therapy.
Analyses were generally performed on an intention-to-treat basis, with all patients being analysed in the groups to which they were randomized. However, owing to clerical errors, two patients in each arm were incorrectly prescribed the alternate drug. As these patients had not received any randomized drug, neither the patient nor clinician was aware of the original allocation, and the two groups remained balanced, these four patients were included in the analysis in their treated groups. All analyses were repeated after including these individuals in their randomized groups with the same conclusions. Missing values were imputed using a last observation carried forward approach, although analyses in which values were not imputed gave similar results. For the analyses of the primary endpoint and other analyses including DEXA/CT scan results, individuals with missing values were excluded, as these scans were performed too infrequently to permit reliable imputation of missing values.
Further pre-planned analyses included an assessment of the change in limb fat mass according to whether the patient was receiving a protease inhibitor or non-nucleoside reverse transcriptase inhibitor, whether they had been receiving zidovudine or stavudine, and whether their baseline fat mass was above or below the median value. These analyses were exploratory as the study was not powered to detect any differences in these subgroups.
To ensure 80% power to detect a 0.5 kg difference in mean limb fat mass between the two arms (with SD of 0.85 kg) at the 5% level of significance, 46 subjects were required in each treatment arm. A dropout rate of 8-10% was assumed and so the study aimed to recruit 50 subjects per arm.
Results
Demographics and subject disposition
Between January and October 2003, 105 individuals were recruited to the study; 52 patients were randomized to tenofovir DF and 53 to abacavir. Patients were well matched for baseline characteristics (Table 1) although a higher proportion of abacavir recipients switched from zidovudine (41%) compared with tenofovir DF recipients (23%) (P = 0.06).
Of the 52 patients on tenofovir at baseline:
median nadir CD4 was 114;
at randomization median CD4 was 486;
median time on ART was 5.7 yrs;
thymidine analogue at randomization: 40 (77%) on d4T, 12 (23%) on AZT; median
DEXA measurement (kg, range):
total fat: 11.1 (4.6-28.6);
limb fat: 3.0 (1.2-9.9);
truncal fat 7.1 (2.1-17.6).
Median adipose tissue by CT (cm3, range):
visceral (150 (96-190);
subcutaneous: 87 (56-151);
total: 237 (191-328).
Median metabolic parameters:
total chol (mmol/l, range): 5.6 (2.6-13.3)
HDL-chol (mmol/l, range): 1.3 (0.67-4.4)
LDL-chol (MMOL/l, range): 3.3 (2.9-4.0)
Triglycerides (mmol/l, range): 2.0 (0.5-22.7)
Insulin (IU/l, range): 8.8 (1.7-52.3)
Glucose: (mmol/l, range):5.1 (3.8-5.9)
Of the 52 patients on abacavir at baseline:
median nadir CD4 was 154;
at randomization median CD4 was 521;
median time on ART was 4.9 yrs;
thymidine analogue at randomization: 31 (58%) on d4T, 22 (41%) on AZT;
median DEXA measurement (kg, range):
total fat: 10.6 (5.5-31.3);
limb fat: 2.9 (1.0-10.8);
truncal fat 6.8 (2.7-19.4).
Median adipose tissue by CT (cm3, range):
Visceral: 148 (110-187);
subcutaneous: 81 (69-110);
total: 241 (192-302).
Median metabolic parameters:
total chol (mmol/l, range): 5.3 (2.3-10.7)
HDL-chol (mmol/l, range): 1.3 (0.7-2.2)
LDL-chol (MMOL/l, range): 3.0 (2.4-3.9)
Triglycerides (mmol/l, range): 1.7 (0.5-16.1)
Insulin (IU/l, range): 7.2 (1.4-55.4)
Glucose: (mmol/l, range): 5.2 (3.5-9.4)
The majority of patients completed 48 weeks of study with only three (6%) tenofovir DF and eight (15%) abacavir recipients discontinuing study (Fig. 1). Drug-related adverse events leading to discontinuation occurred in one patient in the tenofovir DF group (diarrhoea) and three (6%) in the abacavir group (all suspected hypersensitivity). Other reasons for discontinuation are described in Fig. 1. Among those who discontinued, the median time to discontinuation was 36 weeks (range, 4-39) in the tenofovir DF group and 19 weeks (range, 1.6-40) in abacavir recipients.
Only one participant, receiving abacavir, experienced viral rebound. Changes in CD4 cell count did not differ significantly between the two groups over 48 weeks [mean difference 5.9; 95% confidence interval (CI), -75.3 to 87.0; Table 2].
Table 2. Changes in primary (change in limb fat) and secondary endpoints in each treatment group over 48 weeks and difference between the two treatment arms. The p-values did not show significant differences in changes between ABC & TDF regarding DEXA & CT. Chol (p 0.003) and LDL (p 0.04) were the only significant p-values in terms of differences in changes between TDF& ABC regarding metabolics.
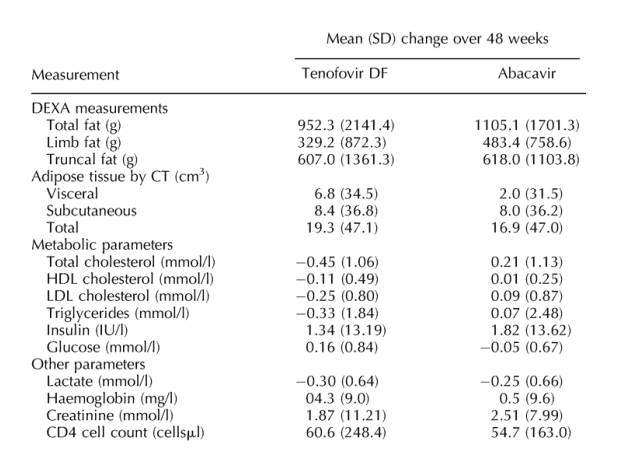
Changes in regional fat mass
Patients were well matched at baseline for total, limb and trunk fat and for visceral and subcutaneous adipose tissue (Table 1). Median limb fat by DEXA increased similarly in both groups from randomization to week 48 (Fig. 2). Tenofovir DF recipients gained a mean 143 g (SD, 646) by week 24 and 329 g (SD, 872) by week 48 (P = 0.01) whereas abacavir recipients gained a mean 206 g (SD, 603) by week 24 and 483 g (SD, 759) by week 48 (P = 0.0001). The difference in change in limb fat mass between the two treatment arms at 48 weeks was not significant (mean difference -154.3; 95% CI, -492.8 to 184.3; Table 2); adjustment for the baseline inbalance in zidovudine use did not modify these findings. Mean changes in trunk and total fat over 48 weeks did not differ significantly between treatment arms.
Persons receiving stavudine before switching experienced greater increases in limb fat than those on zidovudine (Fig. 3). The 37 tenofovir DF recipients receiving stavudine prior to switching had a mean increase in limb fat of 499 g (SD, 749) from a median randomization value of 2.91 kg (range, 1.17-9.60), whereas the 12 taking zidovudine prior to switching had a mean decrease in limb fat of 195 g (SD, 1043) from a randomization value of 5.12 kg (range, 1.58-9.90). The 28 abacavir recipients receiving stavudine before randomization had a mean increase in limb fat of 615 g (SD, 847) from median randomization value of 2.74 kg (range, 0.97-10.78), compared with a mean increase of 253 g (SD, 520) from 2.97 kg (range, 1.91-8.01) among the 16 zidovudine recipients. Use of a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor in the regimen and baseline fat mass did not influence limb fat recovery (Fig. 3).
Both groups experienced modest increases in subcutaneous adipose tissue over 48 weeks and no substantial changes in visceral adipose tissue (Table 2). Total abdominal fat at L4 increased by a median of 25 cm2 (95% CI, -107 to 108) in the tenofovir DF group and 25 cm2 (95% CI, -160 to 96) in the abacavir recipients (P = 0.86).
Lipid parameters
Lipid parameters improved when switching to tenofovir DF but were unchanged after switching to abacavir. Changes in total cholesterol (P = 0.003) and LDL cholesterol (P = 0.04) significantly favoured tenofovir DF relative to abacavir (Table 2).
Lipid parameters were generally higher in persons receiving stavudine at baseline. A decline in median total cholesterol over 48 weeks in those receiving stavudine at entry was observed when switching to tenofovir DF (from 5.9 to 5.5 mmol/l) while no change was seen in those switching to abacavir (5.4 mmol/l at both times). In individuals receiving zidovudine at entry, small rises in median total cholesterol were observed (from 4.9 to 5.1 mmol/l for tenofovir DF and from 5.3 to 5.9 mmol/l for abacavir). In multivariable analyses, both the treatment group (P = 0.002) and receipt of stavudine at baseline (P = 0.02) were independently associated with the change in total cholesterol, although there was no evidence of an interaction between the two (P = 0.98). Using National Cholesterol Education Program cut-offs, the proportion of patients with total cholesterol ≥ 6.2 mmol/l fell in tenofovir DF recipients from 38% at randomization to 25% at week 48 (P = 0.07; McNemar's test) and rose in abacavir recipients from 28% to 36% (P = 0.39). No changes were seen in the proportions of patients with raised LDL cholesterol or low HDL cholesterol values in either group.
Lipid-lowering therapy was commenced during the study in two tenofovir DF recipients (at 85 and 273 days after randomization) and eight abacavir recipients (at median 91.5 days). Sensitivity analyses in which individuals starting lipid-lowering therapy had their week 48 values replaced with the values prior to lipid-lowering therapy reached similar conclusions to the main analyses.
Other laboratory markers TOP
Plasma lactate fell significantly from baseline with no differences between arms (P = 0.71). Haemoglobin rose in tenofovir DF recipients but was unchanged in abacavir recipients (P = 0.04). In a post hoc analysis, it was noted that increases in haemoglobin were observed predominately in individuals switching away from zidovudine. In persons taking zidovudine at baseline, mean haemoglobin increased by 1.14 mg/l (SD,.99) and.61 mg/l (SD,.96) in tenofovir DF and abacavir recipients, respectively.
No significant changes in creatinine were observed during the study. No changes in bone mineral density were noted in either group (Table 2). Osteopenia (T-score < 1.5) was present in 10 (19.2%) individuals in each group at baseline; by week 48, 13 (26.7%) tenofovir DF recipients and 7 (15.9%) abacavir recipients had osteopenia (P = 0.32). No patient experienced bone fractures related to study treatment. No significant changes in systolic/diastolic blood pressure, liver function, amylase, glucose or fasting plasma insulin were observed.
Discussion
Lipoatrophy remains a key obstacle to the continuation of antiretroviral therapy in HIV-infected individuals. While a number of potential interventions for lipoatrophy have been investigated, only substitution of stavudine or zidovudine with abacavir has reliably led to limb fat gain in multiple controlled randomized trials [10-12]. This study establishes tenofovir DF as an alternative to abacavir when switching from a thymidine analogue for lipoatrophy. While limb fat recovery is similar with these two options, tenofovir DF had modest advantages relative to abacavir with regards to lipids and was associated with fewer treatment-related discontinuations. Both switches improved other laboratory parameters, notably lactate, and haemoglobin in individuals discontinuing zidovudine. No unexpected safety concerns regarding either drug were raised during the 48 weeks of the study.
A number of previous studies have assessed switching between nucleoside reverse transcriptase inhibitors to manage lipoatrophy [10-12]. The largest of these (MITOX) showed that, in addition to limb fat gains, switching to abacavir had no significant effect on HIV-1 RNA, fasting lipids or glucose after 24 weeks [10]. Modest but significant increases in limb fat were observed in those switching to abacavir over 2 years (mean increases of 0.39 kg and 1.26 kg at weeks 24 and 104, respectively) [10,15]; the benefits on limb fat mass were largely restricted to individuals switching away from stavudine. Other randomized studies [11,12] confirmed these findings and have suggested that switching therapy may also prevent limb fat loss. Changes in limb fat mass in RAVE are smaller than those reported in previous studies, which may reflect the inclusion of a larger proportion of individuals receiving zidovudine. Changes in limb fat in persons switching away from stavudine were in line with previous studies using either abacavir or tenofovir as the replacement agent [10-12,15-17]. The changes in limb fat observed in this and other studies over 48 weeks are modest compared with the deficit in limb fat at baseline relative to unaffected subjects [14] and hence do generally not lead to substantial resolution of the clinical appearance of lipoatrophy over 48 weeks. Data from the MITOX study indicate that limb fat recovery continues at a similar rate in the second year following switch [15]; thus, the deficit in limb fat declines over time, potentially reducing the severity of the clinical picture.
Switching to tenofovir DF or abacavir has only modest effects on other aspects of lipodystrophy. Visceral adipose tissue did not change substantially in either group. Changes in total and LDL cholesterol, while significantly favouring tenofovir DF, represented only approximately a 5% change from baseline values. Switch to tenofovir DF was also associated with a small decline in HDL although this change did not differ significantly from changes in the abacavir arm. The clinical significance of these changes on future cardiovascular risk is not known. Prospective studies of tenofovir DF in initial therapy have also generally observed smaller increases in total, LDL and HDL cholesterol compared with those seen in thymidine analogue-based regimens [7,13].
The increases in haemoglobin in individuals switching away zidovudine at baseline may be clinically relevant, although evaluation of symptoms related to low haemoglobin levels were not sought.
While renal dysfunction has been reported in individuals receiving tenofovir DF [18-20], no differences in changes in creatinine or graded creatinine elevations were observed. These findings are consistent with 3-year data from a randomized controlled study in treatment-naive individuals, which, like RAVE, included individuals with normal renal function at baseline [7]. The observed frequency of abacavir hypersensitivity of 6% is consistent with previous studies [21,22]. Changes in bone mineral density in RAVE were small and their significance uncertain. Small declines in bone mineral density have been reported in persons commencing tenofovir DF and stavudine [7]. However, prolonged follow-up suggests that initial declines in bone mineral density in tenofovir DF recipients may recover over time [16].
The study has some limitations. First, because of clerical errors, four patients received the incorrect study drug. Although these individuals were included in the analysis as treated the results were similar when these individuals were included as randomized. Second, a small number of patients did not attend for their week 48 DEXA scan. It was felt that a 24-week time interval was too long to impute these values and, therefore, these individuals were excluded from analyses including any DEXA endpoints. Analyses in which these individuals were included with their latest (i.e., week 24 or randomization) values gave similar results.
In summary, switching from a thymidine analogue to either tenofovir DF or abacavir leads to statistically significant improvement in limb fat mass over 48 weeks. Both drugs are generally well tolerated and virologically effective. Peripheral lipoatrophy, when clinically apparent, resolves slowly following treatment switching. Where feasible, consideration should be given to proactive switching away from thymidine analogue to either tenofovir DF or abacavir.
Sponsorship: This study was funded and sponsored by Gilead Sciences.
References
1. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853-860.
[Context Link]
2. Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998; 12:F51-F58.
[Context Link]
3. Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993-2003.
[Medline Link] [CrossRef] [Context Link]
4. Amin J, Moore A, Carr A, French MA, Law M, Emery S, et al. Combined analysis of two-year follow-up from two open-label randomized trials comparing efficacy of three nucleoside reverse transcriptase inhibitor backbones for previously untreated HIV-1 infection: OzCombo 1 and 2. HIV Clin Trials 2003; 4:252-261.
[Medline Link] [CrossRef] [Context Link]
5. Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS 2000; 14:1309-1316.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
6. Dube M, Zachin R, Tebas P, Roubenoff R, Mulligan K, Robbins G, et al. Prospective study of regional body composition in antiretroviral naive subjects randomised to receive zidovudine+lamivudine or didanosine+stavudine combined with nelfinavir, efavirenz or both: A5005s, a substudy of ACTG 384. Antivir Ther 2002; 7:L18.
[Context Link]
7. Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, for the 903 Study Group. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191-201.
[Medline Link] [CrossRef] [Context Link]
8. Shlay JC, Visnegarwala F, Bartsch G, Wang J, Peng G, El-Sadr WM, for the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs abacavir and lamivudine. J Acquir Immune Defic Syndr 2005; 38:147-155.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
9. Moyle G, Sutinen J. Managing HIV lipoatrophy. Lancet 2004; 363:412-414.
[Medline Link] [CrossRef] [Context Link]
10. Carr A, Workman C, Smith DE, Hoy J, Hudson J, Doong N, et al. Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy. JAMA 2002; 288:207-215.
[Medline Link] [CrossRef] [Context Link]
11. John M, McKinnon EJ, James IR, Nolan DA, Herrmann SE, Moore CB, et al. Randomized, controlled, 48-week study of switching stavudine and/or protease inhibitors to combivir/abacavir to prevent or reverse lipoatrophy in HIV-infected patients. J Acquir Immun Defic Syndr 2003; 33:29-33.
[Fulltext Link] [Medline Link] [Context Link]
12. Moyle GJ, Baldwin C, Landroudi B, Mandalia S, Gazzard BG. A 48-week, randomized, open-label comparison of three abacavir-based substitution approaches in the management of dyslipidemia and peripheral lipoatrophy. J Acquir Immun Defic Syndr 2003; 33:22-28.
[Fulltext Link] [Medline Link] [Context Link]
13. Pozniak AL, Gallant JE, DeJesus E, Campo R, Arribas JR, Gazzard B, et al. Superior outcome for tenofovir DF (TDF), emtricitabine (FTC) and efavirenz (EFV) compared to fixed dose zidovudine/lamivudine (CBV) and EFV in antiretroviral naive patients. Third IAS Conference on HIV Pathogenesis and Treatment. Rio de Janeiro, July 2005 [abstract WeOa0202].
[Context Link]
14. HIV Lipodystrophy Case Definition Group. An objective definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet 2003; 361:726-735.
[Medline Link] [CrossRef] [Context Link]
15. Martin A, Smith D, Carr A, Ringland C, Amin J, Emery S, for the Mitochondrial Toxicity (MITOX) Study Group. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. AIDS 2004; 18:1029-1036.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
16. Cassetti I, Madruga JVR, Suleiman JMAH, Zhong L, Enejosa J, Cheng AK. Tenofovir DF (TDF) in combination with lamivudine (3TC) and efavirenz (EFV) in antiretroviral-naive HIV-infected patients: 4-year follow-up. Third IAS Conference on HIV Pathogenesis and Treatment. Rio de Janeiro, July 2005 [abstract WePe6.3C05].
[Context Link]
17. Milinkovic A, Lopez S, Vidal S, Miro O, Fernandez X, Arnaiz J, et al. A randomized open study comparing the effect of reducing stavudine dose vs switching to tenofovir on mitochondrial function, metabolic parameters, and subcutaneous fat in HIV-infected patients receiving antiretroviral therapy containing stavudine. 12th Conference on Retroviruses and Opportunistic Infections. Boston, February 2005 [abstract 857].
[Context Link]
18. Malik A, Abraham P, Malik N. Acute renal failure and Fanconi syndrome in an AIDS patient on tenofovir treatment: case report and review of literature. J Infect 2005; 51:E61-E65.
[Medline Link] [CrossRef] [Context Link]
19. Quimby D, Brito MO. Fanconi syndrome associated with use of tenofovir in HIV-infected patients: a case report and review of the literature. AIDS Read 2005; 15:357-364.
[Medline Link] [Context Link]
20. Jones R, Stebbing J, Nelson M, Moyle G, Bower M, Mandalia S, et al. Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently: a cohort and case-control study. J Acquir Immune Defic Syndr 2004; 37:1489-1495.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
21. DeJesus E, Herrera G, Teofilo E, Gerstoft J, Buendia CB, Brand JD, for the CNA30024 Study Team. Abacavir versus zidovudine combined with lamivudine and efavirenz, for the treatment of antiretroviral-naive HIV-infected adults. Clin Infect Dis 2004; 39:1038-1046.
[Medline Link] [CrossRef] [Context Link]
22. Moyle GJ, DeJesus E, Cahn P, Castillo SA, Zhao H, Gordon DN, for the Ziagen Once-Daily in Antiretroviral Combination Therapy (CNA30021) Study Team. Abacavir once or twice daily combined with once-daily lamivudine and efavirenz for the treatment of antiretroviral-naive HIV-infected adults: results of the Ziagen Once Daily in Antiretroviral Combination Study. J Acquir Immune Defic Syndr 2005; 38:417-425.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
|
|
| |
| |
|
|
|