| |
Tipranavir RESIST-2 Published Results
|
| |
| |
Ritonavir-Boosted Tipranavir Demonstrates Superior Efficacy to Ritonavir-Boosted Protease Inhibitors in Treatment-Experienced HIV-Infected Patients: 24-Week Results of the RESIST-2 Trial
Clinical Infectious Diseases Nov 15, 2006;43:1347-1356
Pedro Cahn,1 Jorge Villacian,5 Adriano Lazzarin,8 Christine Katlama,9 Beatriz Grinsztejn,2 Keikawus Arasteh,7 Paulo Lopez,3 Nathan Clumeck,10 Jan Gerstoft,11 Nikolas Stavrianeas,12 Santiago Moreno,13 Francisco Antunes,14 Dietmar Neubacher,6 and Douglas Mayers4
1Fundacion Huesped, Buenos Aires, Argentina; 2Fundacao Oswaldo Cruz, Rio de Janeiro, Brazil; 3Hospital Juan I Menchaca, Instituto Mexicano del Seguro Social, Guadalajara-Jalisco, Mexico; 4Boehringer Ingelheim Pharmaceuticals, Ridgefield, Connecticut; 5Boehringer Ingelheim GmbH, Germany, 6Boehringer Ingelheim GmbH and Co. KG, Ingelheim, and 7Auguste-Viktoria Klinikum, Berlin, Germany; 8Vila-Salute San Raffaele University, Milan, Italy; 9Hopital de la Pitie-Salpetriere, Paris, France; 10Hopital Universitaire St. Pierre, Bruxelles, Belgium; 11Rigshospitalet, Copenhagen, Denmark; 12Andreas Syngros Hospital, Athens, Greece; 13Hospital Ramon y Cajal, Madrid, Spain; and 14Hospital de Santa Maria, Lisbon, Portugal
(See the article by Gathe et al. on pages 1337-46)
ABSTRACT
Background. Tipranavir, a novel protease inhibitor, has demonstrated antiviral activity against protease inhibitor-resistant human immunodeficiency virus type 1 (HIV-1) isolates. The Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST-2) trial is an ongoing, open-label, phase III trial comparing ritonavir-boosted tipranavir (TPV/r) plus an optimized background regimen with an individually optimized, ritonavir-boosted protease inhibitor in treatment-experienced, HIV-1-infected patients.
Methods. Patients at 171 sites in Europe and Latin America who had received >2 previous protease inhibitor regimens, had triple-antiretroviral class experience, had an HIV-1 RNA level >1000 copies/mL, and had genotypically demonstrated primary protease inhibitor resistance were eligible. After genotypic resistance tests were performed, a protease inhibitor and optimized background regimen were selected before randomization. Patients were randomized to receive either TPV/r or comparator protease inhibitor-ritonavir (CPI/r) and were stratified on the basis of preselected protease inhibitor and enfuvirtide use. Treatment response was defined as a confirmed HIV-1 load reduction >1 log10 less than the baseline value without a treatment change at week 24.
Results.
A total of 863 patients were randomized and treated. At baseline, the mean HIV-1 load was 4.73 log10 copies/mL, and the mean CD4+ cell count was 218 cells/mm3.
The preplanned 24-week efficacy analyses of 539 patients demonstrated treatment response rates of 41% in the TPV/r arm and 14.9% in the CPI/r arm (intent-to-treat analysis; P < .0001).
The mean CD4+ cell count increased by 51 cells/mm3 in the TPV/r arm and by 18 cells/mm3 in the CPI/r arm.
The most common adverse events were mild-to-moderate diarrhea, nausea, and headache.
Grade 3 or greater elevations in serum transaminase, cholesterol, and triglyceride levels were more frequent in the TPV/r arm.
Conclusions. TPV/r had superior antiviral activity and increased immunologic benefits, compared with CPI/r, at week 24 among treatment-experienced patients infected with multidrug-resistant HIV-1.
INTRODUCTION
Combination antiretroviral therapy has resulted in dramatic decreases in morbidity and mortality among patients with HIV-1 infection [1, 2]. However, 30% of patients who initiate antiretroviral therapy do not maintain virologic response, often because of reduced compliance with treatment, adverse drug reactions, and/or development of viral resistance [3-9]. Widespread use of protease inhibitors (PIs) has led to the emergence of resistance to these agents. The rate of PI resistance is reported to range from 30% to 50% among newly infected patients and patients who have already been treated with these agents [10, 11]. Cross-resistance among antiretroviral drugs (including PIs) often limits treatment options and increases morbidity and mortality. Therefore, new agents are needed to respond to limited treatment options [11-13].
Tipranavir is a novel PI with a structure distinct from that of peptidic PIs [14-16]. In vitro studies of clinical HIV-1 isolates have shown that tipranavir remains active against most PI-resistant viruses [17-19]. Tipranavir is coadministered with ritonavir (TPV/r) to assure therapeutic concentrations are attained [20-24], and together, they have demonstrated potent antiviral responses in both treatment-naive and triple drug class-experienced patients [25, 26].
The Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) pivotal trial program comprises 2 almost identical trials, which are being performed in different locations worldwide. RESIST-1 was conducted in North America and Australia [27]. Both RESIST studies are open-label, phase III trials evaluating the efficacy and safety of TPV/r (500 mg/200 mg twice daily) versus an investigator-selected, ritonavir-boosted comparator PI (CPI/r), both with an optimized background regimen. The results of the 24-week interim analyses of RESIST-2 are reported here.
METHODS
Study design. This study is being conducted at 171 sites in Europe and Latin America. The primary end point for the preplanned interim analysis was the proportion of patients who achieved a treatment response at week 24, defined as a >1-log10 reduction in the HIV-1 load from baseline (for 2 consecutive measurements) without prior virologic failure, discontinuation of study drug, introduction of a new drug, loss to follow-up, or death. Secondary end points included change in the HIV-1 load and CD4+ cell count, the proportion of patients who achieved an HIV-1 load <400 or <50 copies/mL, and the number of events indicating progression to AIDS.
Safety end points included any adverse event, laboratory abnormalities, and serious adverse events, including death. The Division of AIDS Intensity Scale (grade 1, mild; grade 2, moderate; grades 3 and 4, severe; and grade 4, severe and serious) was used as a guide to help assess the intensity of adverse events.
Patients. HIV-infected patients who had received >3 months of prior treatment with all 3 classes of antiretrovirals (including 2 PI-based regimens, one being the regimen at screening) and who had a baseline HIV-1 RNA level >1000 copies/mL were eligible for the study. There were no restrictions on CD4+ cell count, although patients had to be free of opportunistic infections or receiving stable treatment. A screening protease genotype with >1 primary PI mutation at codons 30N, 46I/L, 48V, 50V, 82A/F/L/T, 84V, or 90M [13] and <2 key tipranavir mutations at codons 33, 82, 84, or 90 were required for study entry. Grade >2 laboratory values were exclusionary (grade 2 levels were allowed for total cholesterol and triglyceride levels). Exclusion criteria included antiretroviral treatment interruptions that lasted >7 consecutive days within 3 months before the screening, prior tipranavir use, pregnancy, and breast-feeding.
Study participation was voluntary; written informed consent was obtained from all patients. The protocol and informed consent were approved by independent ethics committees or institutional review boards at each center.
Treatment. Before randomization, investigators selected a CPI/r and an optimized background regimen for each patient on the basis of genotypic resistance and antiretroviral medication history. Viral resistance experts (see RESIST-2 Members below) were available to assist with CPI/r selection. Investigators could include a new or previously used PI in the regimen. Enfuvirtide could also be included in the regimen, even if it had been used before randomization.
Patients were randomized to receive TPV/r or the preselected CPI/r (lopinavir boosted with ritonavir, 400 mg/100 mg twice per day; indinavir boosted with ritonavir, 800 mg/100 mg twice per day; saquinavir boosted with ritonavir, 1000 mg/100 mg or 800 mg/200 mg twice per day; or amprenavir boosted with ritonavir, 600 mg/100 mg twice per day). Preselected PI use and enfuvirtide use were stratification criteria for randomization. Tipranavir (250-mg capsules) was supplied by Boehringer Ingelheim; comparator PIs and ritonavir were commercially acquired.
Changes to the background antiretroviral regimen were allowed in cases of toxicity or intolerance. If virologic failure (defined as a decrease in the HIV-1 load of <1 log10 from the baseline level noted by 2 consecutive assays or as failure to achieve an HIV-1 load of <100,000 copies/mL) was documented after 8 weeks of treatment, patients receiving CPI/r were allowed to "roll over" into a companion study in which they could receive TPV/r.
Sample analysis. Plasma HIV-1 RNA levels were measured using Roche Amplicor HIV-1 Monitor Assay, version 1.5 (Roche Diagnostic Systems) or the Roche UltraSensitive Method, version 1.5. CD4+ lymphocyte counts were measured using standard flow cytometry. All tests were conducted by Covance Central Laboratory Services (Geneva, Switzerland). HIV genotypic resistance assessment at screening used Virtual Phenotype, version 3.6 (Virco), for European countries and the HIV-1 genotyping method, version 1.0 (TruGene), for Latin America. A randomly selected subset of patients' isolates was phenotyped post hoc using the Virco Antivirogram assay.
Statistical analysis. The interim 24-week efficacy analyses were performed for all patients who had been randomized for at least 24 weeks as of 19 March 2004. Treatment response at week 24 was analyzed using Cochran-Mantel-Haenszel tests stratified according to preselected PI and enfuvirtide use.
A sample size of 404 patients per treatment arm provided 80% power to detect a 10% difference between the TPV/r arm over the CPI/r arm in treatment response. For the binary efficacy parameters, treatment response and proportion of patients with undetectable HIV-1 loads, intent-to-treat (ITT) analyses classified subjects who did not complete the study as having treatment failure (ITT-NCF). For patients with HIV-1 loads <50 copies/mL, an HIV-1 load value of 49 copies/mL was imputed. Continuous efficacy end points (HIV-1 load and CD4+ cell count) were analyzed using the last observation carried forward method. All randomized patients who received 1 dose of study medication were included in the safety population, and data were analyzed using descriptive statistics.
RESULTS
Baseline patient characteristics. In total, 863 patients were randomized and received 1 dose of study treatment. The 24-week efficacy analyses included 539 patients. Demographic characteristics of the full analysis set were comparable for both groups (table 1).
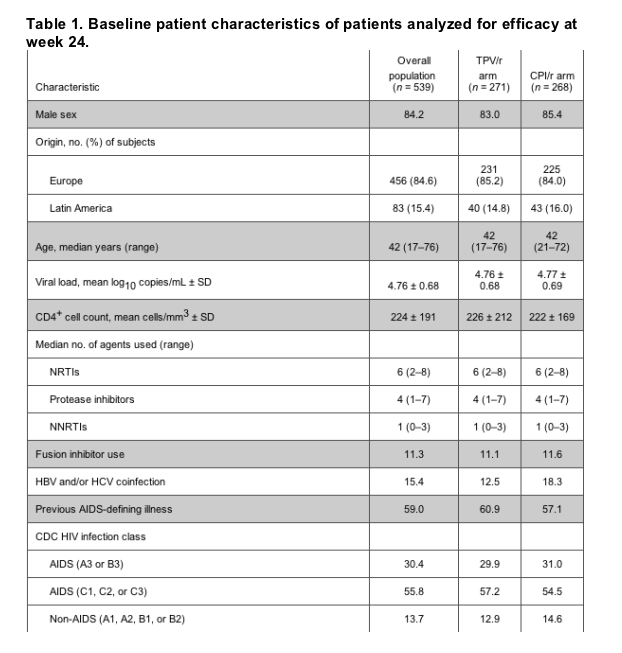
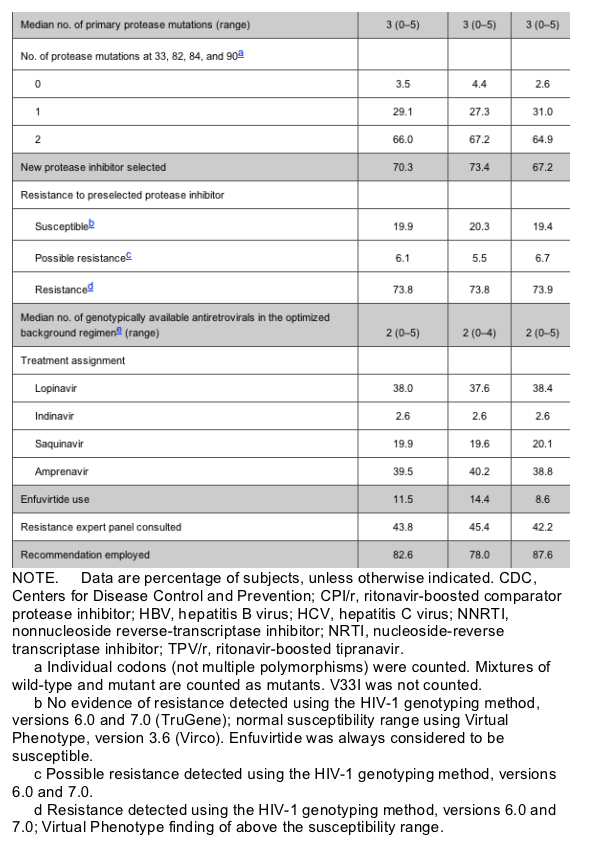
Baseline resistance characteristics. Baseline PI resistance was comparable between the 2 arms, with a median of 3 primary protease gene mutations (30N, 46I/L, 48V, 50V, 82A/F/L/T, 84V, or 90M) in each group. The median baseline phenotypic fold-change in susceptibility to tipranavir, assessed retrospectively in a randomly selected sample of virus isolates recovered from 225 patients (199 patients from Europe and 26 patients from Latin America), was 1.4 times the IC50 for wild-type virus. The median fold-changes in IC50 for the comparator PIs in these isolates were 104-fold for lopinavir, 45.4-fold for indinavir, 15.5-fold for saquinavir, and 11.8-fold for amprenavir.
The comparator PI selected was amprenavir in 39.5% of patients, lopinavir in 38.0%, saquinavir in 19.9%, and indinavir in 2.6%. The most commonly prescribed optimized background regimen (for 39.9% of patients) included 2 nucleoside reverse-transcriptase inhibitors. The preselected optimized background regimen (including enfuvirtide, which is always considered to be genotypically susceptible) contained a median of 2 active (susceptible or partially susceptible in the screening genotype interpretation) antiretroviral drugs.
Patient disposition. Significant differences between the groups in favor of TPV/r were noted for all patients who discontinued treatment and for those who discontinued specifically because of virologic failure (P < .0001 for both) (figure 1).
Treatment response. TPV/r-treated patients demonstrated a significantly higher treatment response at week 24 (for the ITT-NCF population) than did CPI/r-treated patients (41.0% vs. 14.9%; P < .0001) (figure 2A). The difference in response rates, adjusted for comparator PI and enfuvirtide use, was 25.0% (95% CI, 17.8%-32.2%). TPV/r demonstrated a superior treatment response in each comparator PI stratum (table 2).
The rate of treatment response to TPV/r increased from 21.1% (when the optimized background regimen contained no drugs to which the genotype was susceptible) to 54.7% (with the addition of 3 drugs to which the genotype was susceptible), compared with an increase from 0% to 29.8% in the CPI/r arm. The use of enfuvirtide was associated with an improved response rate in the TPV/r arm (from 37.9% to 59.0%; P = .0136) but not in the CPI/r arm (15.5% vs. 8.7%).
Secondary efficacy end points. The mean reduction from baseline in the HIV-1 load was significantly greater in the TPV/r arm than in the CPI/r arm (-1.23 vs. -0.53 log10 copies/mL; P < .0001), as shown in figure 2B-D. More patients achieved an undetectable HIV-1 load in the TPV/r arm than in the CPI/r arm (for <400 copies/mL, 33.6% vs. 13.1%; for <50 copies/mL, 22.5% vs. 8.6%; P < .0001 for both). Among TPV/r-treated patients, the proportion of patients who achieved an HIV-1 load <400 copies/mL increased from 32.8% among those who did not receive enfuvirtide to 38.5% among those who did receive enfuvirtide (P = .4492), whereas it had no effect in CPI/r-treated patients. Enfuvirtide use did not increase the proportion of patients with an HIV-1 load <50 copies/mL in either arm. The mean increase from baseline in the CD4+ cell count was significantly higher in the TPV/r arm than in the CPI/r arm (increases of 51 vs. 18 cells/mm3; P < .0001).
Overall, 14 (3.2%) and 18 (4.2%) of the patients in the TPV/r and CPI/r arms, respectively, experienced events indicative of progression to AIDS (both new and recurrent events). The number of deaths during the study was similar between treatment arms (4 [0.9%] and 5 [1.2%] in the TPV/r and CPI/r arms, respectively); all deaths were associated with AIDS-related illnesses in the context of severe immunosuppression.
Resistance analyses. Treatment response with TPV/r was superior to treatment with CPI/r, regardless of the number of primary PI gene mutations. For patients who were infected with viruses harboring 1-2 primary mutations (201 patients; 97 in the TPV/r arm and 104 in the CPI/r arm), the rate of virologic response was 42.3% in the TPV/r arm compared with 21.2% in the CPI/r arm. With 3-4 primary mutations (326 patients; 166 in the TPV/r arm and 160 in the CPI/r arm), the rates of virologic response were 41% in the TPV/r arm versus 11.3% in the CPI/r arm; finally, with 5-6 mutations (9 patients; 6 in the TPV/r arm and 3 in the CPI/r arm), responses were achieved in 33.3% of cases in the TPV/r arm, compared with none in the CPI/r arm.
As baseline phenotypic resistance to TPV increased, treatment response decreased. Of the 225 virus isolates phenotyped, 179 were recovered from patients in the TPV/r arm. Of 58 TPV/r-treated patients who had a <1-fold change in the tipranavir IC50 from baseline, 32 (55.2%) had treatment responses at 24 weeks. Treatment response diminished as the IC50 fold-changes increased; 1-2-fold changes in the IC50 occurred in 50 patients, 2-3-fold changes occurred in 29 patients, 3-4-fold changed occurred in 12 patients, 4-8-fold changes occurred in 14 patients, and >8-fold changes occurred in 16 patients, with corresponding treatment responses of 34.0%, 41.4%, 33.3%, 21.4%, and 12.5%, respectively.
Safety. Overall, most adverse events were of mild or moderate intensity, and the rate of adverse events (not adjusted for exposure) was higher in the TPV/r arm (table 3). Treatment-related serious adverse events were infrequent in both treatment arms. Of the 863 patients in the safety population, 30 (6.9%) and 20 (4.7%) discontinued TPV/r and CPI/r therapy, respectively, because of adverse events, which were mostly gastrointestinal in nature (11 and 8 patients, respectively).
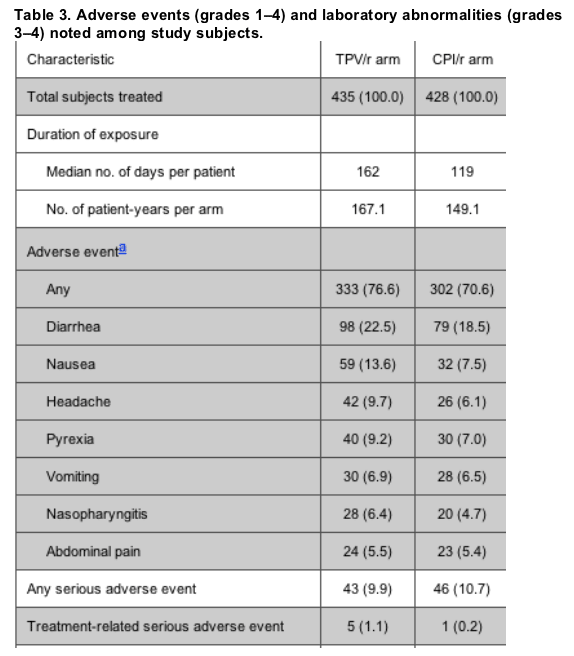
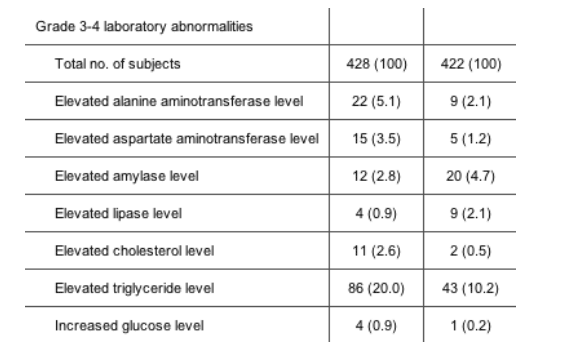
Grade 3/4 laboratory abnormalities were infrequent and were observed in >5% of TPV/r-treated patients for alanine aminotransferase and triglycerides (table 3). In the TPV/r arm, 8 (36.4%) of 22 patients with grade 3/4 elevations in the alanine aminotransferase levels continued taking the study drug. In addition, 8 patients (36.4%) temporarily discontinued TPV/r use until the alanine aminotransferase level normalized, and 6 (27.3%) discontinued TPV/r use permanently. Most grade 3/4 abnormalities in the alanine aminotransferase level occurred in patients with preexisting grade 1 elevations in the alanine aminotransferase level, and the risk of developing grade 3/4 elevations in the alanine aminotransferase level was higher among patients coinfected with hepatitis B or C virus. The overall frequency of grade 3/4 elevations in the alanine aminotransferase level among patients with hepatitis B was 4.8% (18 of 373 patients), compared with 7.3% (4 of 55) among patients in the TPV/r arm who had hepatitis B or C (P = .4726).
DISCUSSION
The use of TPV/r in a population of treatment-experienced patients was associated with a superior treatment response, compared with the use of CPI/r, over a 6-month period across all PI strata. The mean reduction from baseline in HIV-1 load for the TPV/r arm versus the CPI/r arm was both statistically and clinically significant. Moreover, significantly more patients in the TPV/r arm achieved HIV-1 loads that were less than the limit of detection. Although the RESIST-2 study was not powered to show a difference in clinical outcomes, the reductions in the HIV RNA level that were achieved are likely to have clinical benefits, because reductions in HIV RNA levels of >0.5 log from the baseline level, as observed here among TPV/r recipients, are associated with a decreased risk of progression to AIDS [28-30].
The goal of treatment in HIV-1-infected patients is to achieve maximal suppression of viral replication and to increase the probability of more-durable treatment responses, delaying development of further drug resistance. Combined with the superior virologic response, patients in the TPV/r arm also had a significant increase in CD4+ T cell count. Increases in CD4+ cell counts of this magnitude have been associated with improved clinical outcomes [31].
Treatment regimens containing a combination of active antiretrovirals are the current standard of care. For patients for whom several regimens have failed, selection of a viable combination is challenging because of increasing drug resistance; however, as demonstrated here, the use of other antiretrovirals to which the virus demonstrates genotypic susceptibility enhances the rate of response to TPV/r from 21.1% (with no active drugs) to 54.7% (when >3 genotypically available drugs were included in the "backbone"). Enfuvirtide was the most likely candidate for a fully active background medication, because the majority of patients were enfuvirtide naive. As expected, treatment responses improved further, to 59% among patients who used enfuvirtide with TPV/r. Interestingly, fewer patients in the CPI/r arm who received enfuvirtide achieved treatment responses, compared with those who did not use enfuvirtide. Although the explanation for this observation is unclear, it could be attributed to the additive effect of administering enfuvirtide with another new drug (tipranavir); however, further evaluations are warranted.
RESIST-2 was designed to minimize patient risk while investigating the antiviral activity and safety of TPV/r, compared with standard-of-care CPI/r regimens. For the patient population addressed in this study, the standard of care is individualized therapy that accounts for prior antiretroviral treatment history and resistance testing. The presence of 1 primary protease mutation ensured that patients would have some resistance to PIs, and limiting the number of key tipranavir mutations improved the probability of response to both tipranavir and any given CPI. To ensure that patients received the best possible PI-based therapy available, rather than choosing a fixed comparator PI, 4 CPI/r options were allowed for selection by the investigator. Antiviral-resistance expert input (from the Resistance Panel) was available to assist with the choice of optimized background regimen and the best possible CPI/r regimen. In addition, enfuvirtide use was permitted if the investigator declared this prior to randomization. Finally, after 8 weeks of treatment, patients in the CPI/r group with demonstrated virologic failure could "roll over" to a companion trial in which they received TPV/r. Provisions were taken to ensure that patients who switched to the companion trial had detectable levels of comparator PIs and had met the criteria for treatment failure or nonresponse. The "roll over" option made it difficult to highlight a clinical benefit among tipranavir recipients, because patients who were most likely to experience disease progression could leave the comparator arm early. However, we believe that the most ethical way to conduct clinical trials that involve this population is to offer patients with few treatment options access to a new and potentially active drug.
Overall, adverse events were more frequent in the TPV/r arm than in the CPI/r arm, but few patients discontinued study medication. Conversely, serious adverse events were more frequent in the CPI/r arm. Safety profile comparisons between TPV/r and CPI/r in RESIST-2 are difficult. The trial was open-label in design because of the complexities of blinding the study treatments, which would have resulted in an unacceptable pill burden. As a result, there is a possible bias toward the reporting of more adverse effects in the TPV/r arm [32]. In addition, drug exposure differs between the 2 arms, because patients who experienced treatment failure in the CPI/r arm could "roll over" to the companion TPV/r trial after week 8. Adjustment for treatment exposure resulted in a similar overall rate of adverse events.
In RESIST-2, elevations in levels of serum transaminases (alanine and aspartate aminotransferase), triglycerides, and cholesterol occurred more frequently in the TPV/r arm. Risk factors for development of these abnormalities included elevated baseline values at study entry and, in the case of alanine and aspartate aminotransferase levels, coinfection with hepatitis B or C virus. Importantly, of 22 patients who developed elevations in transaminase levels, 16 did not discontinue TPV/r treatment.
A higher daily dose of ritonavir was used in the TPV/r arm (400 mg/day) than in the CPI/r arm (200 mg/day). Previously, it has been shown that trough ritonavir concentrations vary considerably in HIV-infected patients receiving ritonavir-boosted PIs with an optimized background regimen [33]. Average ritonavir trough concentrations reported in the TPV/r and amprenavir-ritonavir groups were lower than those observed in the lopinavir-ritonavir and saquinavir-ritonavir groups [33]. This may explain why the tolerability profile of TPV/r is similar to that of other ritonavir-boosted PIs.
In summary, our findings strongly support TPV/r use in HIV-1-infected patients who are treatment experienced (i.e., have received >3 antiretroviral classes) and for whom >2 PI-containing regimens have failed. The superior antiviral activity of TPV/r plus an optimized background regimen, compared with the investigator-chosen CPI/r regimen, was demonstrated by virologic and immunologic surrogate markers that correlate with improved clinical outcomes. Furthermore, the safety profile of TPV/r was comparable with that of other ritonavir-boosted PIs, with the exception of manageable hepatic transaminase and serum lipid abnormalities. In clinical practice, TPV/r may offer the opportunity of therapeutic success to patients who are infected with drug-resistant HIV-1 strains.
|
|
| |
| |
|
|
|