| |
Rosiglitazone Increased Subcutaneous Fat
|
| |
| |
"Effect of Rosiglitazone on Insulin Sensitivity and Body Composition in Type 2 Diabetic Patients"
Obesity Research 10:1008-1015 (2002)
David G. Carey*, Gary J. Cowin, Graham J. Galloway, Nigel P. Jones, Jackie C. Richards, Nandita Biswas and David M. Doddrell
* Wesley Research Institute, Brisbane, Australia,
Centre for Magnetic Resonance, University of Queensland, Brisbane, Australia,
GlaxoSmithKline, Harlow, United Kingdom, and
GlaxoSmithKline, Collegeville, Pennsylvania.
"....This study supports previously presented data regarding euglycemic hyperinsulinemic clamp for RSG and provides evidence that RSG considerably improves insulin sensitivity in patients with type 2 diabetes (23). The improvement in insulin sensitivity was associated with a significant increase in subcutaneous but not visceral adiposity and a decrease in tissue (liver) triglyceride levels....A proportion of the increase in body fat with RSG treatment may be directly attributable to improved glycemic control....Our DXA and MRI data demonstrate that after RSG treatment, an increase in subcutaneous adipose tissue occurs with no increase in intra-abdominal fat. This supports the concept that RSG has depot-specific actions on adiposity....In other diabetic study populations, 12 weeks of treatment with either troglitazone (31) or pioglitazone (32) was associated with an increase in abdominal subcutaneous fat and a significant decrease in MRI-measured intra-abdominal-fat mass. Our data therefore support both in vitro (8) and in vivo studies (28) (31) , and suggest that, like other TZDs, RSG treatment is associated with the uptake of lipid into subcutaneous but not visceral adipose tissue...."
Abstract
Objective: To investigate the effects of rosiglitazone (RSG) on insulin sensitivity and regional adiposity (including intrahepatic fat) in patients with type 2 diabetes.
Research Methods and Procedures: We examined the effect of RSG (8 mg/day, 2 divided doses) compared with placebo on insulin sensitivity and body composition in 33 type 2 diabetic patients. Measurements of insulin sensitivity (euglycemic hyperinsulinemic clamp), body fat (abdominal magnetic resonance imaging and DXA), and liver fat (magnetic resonance spectroscopy) were taken at baseline and repeated after 16 weeks of treatment.
Results:
There was a significant improvement in glycemic control (glycosylated hemoglobin -0.7 ± 0.7%, p 0.05) and an 86% increase in insulin sensitivity in the RSG group (glucose-disposal rate change from baseline: 17.5 ± 14.5 μmol glucose/min/kg free fat mass, p < 0.05), but no significant change in the placebo group compared with baseline.
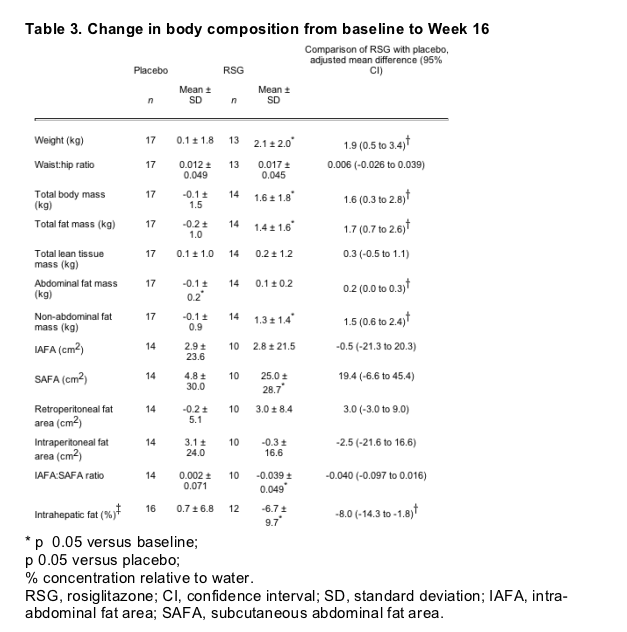
Total body weight and fat mass increased (p 0.05) with RSG (2.1 ± 2.0 kg and 1.4 ± 1.6 kg, respectively) with 95% of the increase in adiposity occurring in nonabdominal regions.
In the abdominal region, RSG increased subcutaneous fat area by 8% (25.0 ± 28.7 cm2, p = 0.02), did not alter intra-abdominal fat area, and reduced intrahepatic fat levels by 45% (-6.7 ± 9.7%, concentration relative to water).
Discussion: Our data indicate that RSG greatly improves insulin sensitivity in patients with type 2 diabetes and is associated with an increase in adiposity in subcutaneous but not visceral body regions.
Introduction
Rosiglitazone (RSG) is a potent member of the thiazolidinedione (TZD) class of oral agents that improve insulin action. TZDs activate nuclear peroxisome proliferator-activated receptor y(PPARy), which is expressed predominantly in adipose tissue. Activation of PPAR promotes the expression of lipogenic genes, resulting in pre-adipocyte growth and differentiation; consequently, total body adiposity may increase (1). This presents a paradox because an increase in body fat is usually associated with insulin resistance. Insulin resistance, however, is closely linked to central rather than peripheral obesity (2). An increase in visceral fat leads to increased lipid availability in the portal (3) and systemic circulation, which in turn is associated with increased intrahepatic (4) (5) (6) and intramuscular lipids (7).
Studies ex vivo have indicated that the adipogenic effect of RSG may be depot-specific, stimulating lipid uptake in subcutaneous but not omental preadipocytes (8). This effect in vivo may result in the channeling of lipids away from visceral areas and toward subcutaneous adipose tissue, a process described as "triglyceride steal" (9). The net result may be a reduction in portal and systemic fatty acid levels and tissue lipotoxicity, a concept that is supported by animal studies in which treatment with RSG resulted in a reduction in circulating fatty acids and intrahepatic fat (10) (11).
This study was designed to investigate the effects of RSG on insulin sensitivity and regional adiposity (including intrahepatic fat) in patients with type 2 diabetes. Insulin sensitivity was determined using a euglycemic hyperinsulinemic clamp (12) , and recently available noninvasive and validated techniques were used to measure fat distribution [magnetic resonance imaging (MRI) and DXA] and intrahepatic lipids [magnetic resonance spectroscopy (MRS)].
Research Methods and Procedures
Patients
Thirty-three white patients with type 2 diabetes (27 men, 6 women) were randomized to receive double-blind medication of RSG (8 mg/day, 2 divided doses) or placebo. Patients were 40 to 80 years of age, with a fasting plasma glucose (FPG) 7 to 15 mM after 2 weeks of placebo run-in, and had a body mass index >22 to <38 kg/m2 at screening. Patients treated by diet and exercise alone and patients that could be withdrawn from low-dose metformin were eligible to enter the study. The protocol was approved by the University of Queensland Research Ethics Committee and informed written consent was obtained from each patient before entering the study, which was performed in accordance with the Declaration of Helsinki and Good Clinical Practice.
Procedures
The study was performed at the Department of Medicine and the Centre for Magnetic Resonance, University of Queensland, Australia. Eligible patients stopped any anti-diabetic therapy and began a 4-week, single-blind, placebo run-in period. Patients were then randomized in equal numbers (stratified by gender) to receive RSG or placebo. Throughout the study, patients were instructed to follow a weight-maintenance diet. An assessment of their dietary intake was made at baseline, at each subsequent study visit, and at the end of the study. Each patient's understanding of and compliance with his or her weight-maintenance diet were also evaluated at these visits.
Assessments of insulin sensitivity (euglycemic hyperinsulinemic clamp) and body composition [MRI, MRS, DXA, body weight, height, waist (smallest circumference between rib and iliac crest), and hips (largest circumference of buttocks)] were performed after the 4-week placebo run-in (baseline measurements) and repeated after 16 weeks of treatment on double-blind medication. Laboratory assays [glycosylated hemoglobin (HbA1c), FPG, fasting plasma insulin, triglycerides, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, apolipoprotein B (ApoB), and free fatty acids (FFA)] were also measured at baseline and repeated at the end of the study.
Clamp Measurements of Insulin Sensitivity
Hyperinsulinemic euglycemic clamp measurements were performed after an overnight fast (12). Insulin was increased for 180 minutes using a primed continuous infusion (50 mU/kg/h Actrapid HM; NovoNordisk A/S, Bagsvaerd, Denmark). A variable-rate glucose infusion was then used to maintain the blood glucose concentration at a steady-state value of 4.5 mM. The glucose-infusion rate at steady-state provided an index of whole-body insulin sensitivity. A metabolic monitor and hood (respiratory gas exchange) was used during the last 30 minutes of the clamp to measure insulin-stimulated nonoxidative glucose disposal. The insulin sensitivity index was calculated as glucose infusion rate at steady-state/insulin value at steady-state.
DXA Measurement of Fat Distribution
Whole-body DXA scanning was performed using a Hologic QDR 4500A machine (Hologic Inc., Bedford, MA) to determine the following fat distribution parameters: total body mass, total lean-tissue mass, total fat-tissue mass, percentage of total-body fat, arm fat, leg fat, trunk fat, abdomen fat mass (from the top of the second to the bottom of the fourth lumbar vertebrae), peripheral fat mass (leg plus arm fat mass), and nonabdominal-fat mass (total fat minus abdominal-fat mass).
MRI Measurement of Abdominal Fat
Abdominal MRI studies were performed using a respiratory gated rapid acquisition with relaxation enhancement (RARE) (Fast Spin Echo) sequence. Images with a resolution of 128 x 128 were acquired with the following parameters: echo-train length = 4; echo time (TE) = 13 ms, giving an effective TE of 32.5 ms; and repetition time determined by the breathing rate and one acquired mean. Four 8-mm slices commencing at the level of the L4-L5 disk and extending rostrally, acquired with and without water suppression, were analyzed. The analysis was based on previously published studies showing that fat areas determined from slices in this region are representative of total abdominal-fat volumes (18) (19). Results from four slices were averaged to minimize potential variability in the positioning of the slices. Intra-abdominal-fat area (IAFA) and subcutaneous abdominal-fat area (SAFA) were measured in each slice by fitting a spline curve to points on the border of the subcutaneous and visceral regions selected by the operator as described previously (20). Nonfat regions within the visceral region were also outlined with a spline fit and subtracted from the total visceral region. The IAFA was subdivided into retroperitoneal and intraperitoneal areas using the ascending and descending colon, the psoas muscles on each side of the spine, and the top of the vessels above the vertebrae, as a guide for the spline fit (20). Corresponding regions from each of the four slices were averaged and presented as areas (in square centimeters).
MRS Measurement of Intrahepatic Fat
Changes in liver fat (percentage of concentration relative to water) were evaluated by MRS. This measurement of hepatic fat has previously been validated against the lipid content of liver biopsies in humans and animals (21) (22). Transverse scout images were acquired through the liver. A 3 x 3 x 3 cm voxel was positioned in the right lobe of the liver to avoid major vessels, and a respiratory gated 1H spectrum was acquired as described previously (21). The volume selective multipulse spin-echo spectroscopy (stimulated echo acquisition mode) acquisition method was used with the following parameters: TE = 18 ms, mixing time = 40 ms, acquisition size = 2000 data points, spectral width = 2000 Hz, number of averages = 16.
Statistical Evaluation
Patients with evaluable data at both baseline and at the end of treatment were included in the data analysis for each endpoint.
The mean changes (± SD) from baseline to the end of treatment have been presented for each treatment group (in Tables 2 and 3 in "Results"). In addition, paired Student's t tests were performed. An analysis of covariance model adjusting for baseline and gender strata was used to compare differences between treatments. The percentage change from baseline was calculated from the geometric mean.
Statistical evaluation of the lipid results (triglycerides, HDL-cholesterol, FFA, LDL-cholesterol:ApoB ratio) was based on the log-transformed data. Summary statistics were transformed back to the original scale and presented as the geometric mean and percentage change from baseline.
Owing to technical issues, four patients (two in the RSG treatment group and two in the placebo group) did not have evaluable MRI data but did have evaluable data for all other endpoints. In addition, the MRS data for intrahepatic fat was not evaluable for one patient in the placebo group.
Results
The two treatment groups were well matched for baseline body mass index, age, duration of diabetes, gender, HbAlc, lipids, insulin sensitivity, and body composition.
Glycemic control (HbA1c and FPG) significantly (p 0.05 vs. baseline and placebo) improved with RSG treatment but not with placebo (Table 2). Fasting plasma insulin levels decreased in the RSG group (-63.5 pM) and increased in the placebo group (2.6 pM) compared with baseline. RSG treatment was related to an 86% increase in insulin sensitivity compared with baseline. The RSG treatment-related increase from baseline in the glucose disposal rate was 17.5 ± 14.5 μmol/glucose/min/kg free fat mass (p < 0.05) compared with an increase of 3.3 ± 9.2 μmol/glucose/min/kg free fat mass (not significant) in the placebo group (Table 2). There was a statistically significant increase in insulin sensitivity index in the RSG group compared with placebo (p = 0.0002) equivalent to an increase of 0.29 ± 0.29 μmol glucose/min/kg free fat mass/mU/L (p = 0.002), and 0.001 ± 0.16 μmol glucose/min/kg free fat mass/mU/L (not significant) in the placebo group compared with baseline (Table 2). The change from baseline in the insulin sensitivity index in the RSG group was 91%. The increase in insulin-stimulated glucose uptake in the RSG group was predominantly due to an increase in nonoxidative glucose disposal (Table 2).
Lipid changes often associated with insulin resistance improved with RSG treatment. HDL-cholesterol increased by 17.5% [95% confidence interval (CI): 7.7, 28.2] in the RSG group compared with 11.0% (95% CI: 3.4, 19.2) in the placebo-treated group. Circulating FFA decreased by 19.3% (95% CI: -39.9, 8.4) in the RSG group and increased in the placebo group by 2.4% (95% CI: -17.7, 27.4). Triglyceride levels decreased by 2.9% (95% CI: -24.7, 25.4) in the RSG group and increased by 7.1% (95% CI: -6.7, 22.9) in the placebo group. The LDL:ApoB ratio increased by 25 from baseline compared with an increase of 0.2 in the placebo group. The change in the RSG-treated patients was consistent with an increase in LDL particle size.
Table 3 shows the changes in fat distribution after 16 weeks of treatment with placebo or RSG. Body weight increased significantly in the RSG treatment group, compared with baseline (p = 0.002) and placebo (p = 0.01) (Table 3). DXA assessments indicate that whereas total fat mass increased by 1.4 kg after treatment with RSG, the increase in lean-tissue mass (water, lean tissue) was nonsignificant. The increase in body fat was not uniform, with 95% of the increase occurring in peripheral or nonabdominal body regions (Table 3).
The MRI data indicate that the increase in fat in the abdominal region occurred in the subcutaneous but not visceral adipose tissue. In the RSG group, there was a significant increase (p = 0.02; 8%) in SAFA compared with baseline accompanied by a significant (p = 0.03) reduction in the IAFA:SAFA ratio (Table 3). No increase in IAFA was observed and there was no significant difference from baseline after separate analysis of intraperitoneal and retroperitonal fat areas in either treatment group (Table 3). Treatment with RSG significantly (p = 0.04) decreased the mean concentration of lipid within the liver from 22% to 15% (a 45% reduction from baseline). There was a small nonsignificant increase in intrahepatic fat in the placebo group, and the difference between treatment groups was statistically significant (p = 0.01) (Table 3). There was no correlation between the change from baseline in insulin sensitivity and liver fat observed in the RSG group (r = 0.012; glucose disposal rate and intrahepatic fat).
Discussion
This study supports previously presented data regarding euglycemic hyperinsulinemic clamp for RSG and provides evidence that RSG considerably improves insulin sensitivity in patients with type 2 diabetes (23). The improvement in insulin sensitivity was associated with a significant increase in subcutaneous but not visceral adiposity and a decrease in tissue (liver) triglyceride levels.
Previously published results obtained using the homeostasis model assessment technique have demonstrated that RSG decreases insulin resistance and improves β-cell function in patients with type 2 diabetes (24). Our results demonstrate that RSG monotherapy improved glycemic control through a significant increase in insulin sensitivity compared with placebo. After treatment with RSG, reductions were also observed in other components of the insulin resistance syndrome including fasting plasma insulin and FFAs.
As with other agents that improve glycemic control, RSG therapy is associated with an increase in body weight. Increased plasma volume and edema has been reported in clinical trials with TZDs (25) (26). The DXA data reported in this study indicate that most of the increase in body mass observed in the RSG group is accounted for by an increase in fat mass. The increase in nonfat tissue mass (water, supportive tissue, muscle, etc.) was not significant, which would suggest that the change in fluid mass observed after treatment with RSG was small and not clinically significant.
A proportion of the increase in body fat with RSG treatment may be directly attributable to improved glycemic control. For example, in the United Kingdom Prospective Diabetes Study, intensive control was accompanied by weight gain of a similar magnitude (27). TZDs, however, are known to directly stimulate lipogenic genes and increase adipose tissue mass, although recent studies indicate that not all adipose tissue is equally responsive to this stimulation (28) (29). When human subcutaneous preadipocytes were incubated with RSG, differentiation was markedly enhanced (8). By contrast, omental preadipocytes (taken from the same individuals) were refractory to RSG, even though PPAR was expressed at similar levels in both depots (8). Our DXA and MRI data demonstrate that after RSG treatment, an increase in subcutaneous adipose tissue occurs with no increase in intra-abdominal fat. This supports the concept that RSG has depot-specific actions on adiposity. It is also of interest that TZDs have been shown to stimulate brown adipose tissue in rodents (30) and our MRI data showed a very small, nonsignificant increase in retroperitoneal fat.
A number of MRI and computed tomography studies investigating the effects of TZDs on body composition have been published recently. For example, Mori et al. (28) report that abdominal subcutaneous fat but not visceral-fat area (measured with computed tomography at the level of the umbilicus) increased in diabetic patients after 6 months of troglitazone treatment. In other diabetic study populations, 12 weeks of treatment with either troglitazone (31) or pioglitazone (32) was associated with an increase in abdominal subcutaneous fat and a significant decrease in MRI-measured intra-abdominal-fat mass. Our data therefore support both in vitro (8) and in vivo studies (28) (31) , and suggest that, like other TZDs, RSG treatment is associated with the uptake of lipid into subcutaneous but not visceral adipose tissue.
31. Kelly, IE, Han, TS, Walsh, K, Lean, ME. (1999) Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes Diabetes Care 22,288-293[Abstract]
32. Miyakawi, Y, Mahankali, A, Matsuda, M, et al (2000) Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in patients with type 2 diabetes [Abstract] Diabetes Res Clin Pract 50(Suppl 1),301
Fatty liver and nonalcoholic steatohepatitis are recognized as part of the insulin resistance syndrome (5) (33) and lesser degrees of fat accumulation may also have clinical significance to type 2 diabetes (34) (35). In rats fed on a high-fat diet, triglyceride accumulation in the liver was reported to be accompanied by the development of liver insulin resistance (the sensitivity of endogenous glucose production to insulin) (11). In addition, liver fat (percentage of liver fat measured by MRS) in diabetic patients has been shown to be closely correlated (r = 0.77, p < 0.001) with liver insulin resistance, more so than any of the other measures of adiposity reported in the study (e.g., percentage of body fat, subcutaneous fat volume, visceral fat volume, total fat mass) (4). The same authors reported that hepatic fat was the parameter most closely correlated with the daily insulin dose of the patients studied (r = 0.82, p < 0.001).
In our study, RSG treatment was associated with a statistically significant decrease in intrahepatic fat (percentage of liver fat), which was not observed in the placebo group. RSG has been shown to reduce levels of liver fat in high-fat fed rats (36) , reduce the degree of hepatocellular fatty change (10) , and reverse hepatic steatosis in Zucker rats (37).
It has been proposed that fatty infiltration into the liver may be related to increased visceral adipose tissue, increased serum triglyceride levels, and insulin resistance (34). Although no change in IAFA was noted in our study, a decrease in the level of circulating FFA was observed after treatment with RSG. It is therefore possible that the reduction in liver fat observed in the RSG group is a consequence of the channeling of lipid away from visceral areas and toward subcutaneous adipose tissue. The net result of this "triglyceride steal" process (9) may be a reduction in portal and systemic fatty acid levels and tissue lipotoxicity. The reduction in liver fat that we report after treatment with RSG may be an important component in the increase in insulin sensitivity associated with this drug. No clear relationship between the change from baseline in insulin sensitivity and liver fat was observed within this relatively small dataset. It will be interesting to study this possibility further in larger studies.
Alternatively, the observed effects of RSG might be mediated by adipocyte secretory products other than FFA, such as tumor necrosis factor- (TNF) or adiponectin. Elevated concentrations of TNF are known to induce insulin resistance, but this action is blocked by RSG both at the level of TNF-altered gene expression and lipolytic activity (38). Type 2 diabetes is associated with low levels of the hormone adiponectin, which has been correlated positively with insulin sensitivity, as measured by euglycemic hyperinsulinemic clamp (39). Recently, RSG has been shown to substantially increase adiponectin levels in patients with type 2 diabetes (40). Whether TNF or adiponectin are biomarkers for RSG-induced improvements in insulin sensitivity or play a more causative role remains to be established.
In conclusion, these results indicate that RSG significantly improves insulin sensitivity and lowers blood glucose in individuals with type 2 diabetes. Although the mechanism of these effects are unclear, it may be due, in part, to the reduction in circulating and tissue (liver) fatty acids that results from an increased uptake and storage of lipid by subcutaneous adipose tissue.
|
|
| |
| |
|
|
|