| |
Longitudinal Anthropometric Changes in HIV-Infected and HIV-Uninfected Men
|
| |
| |
[Epidemiology and Social Science]
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 43(3) 1 November 2006 pp 356-362
Brown, Todd MD*; Wang, Zhaojie BS*; Chu, Haito MD, PhD*; Palella, Frank J MD; Kingsley, Lawrence PhD; Witt, Mallory D MD; Dobs, Adrian S MD, MHS*
From the *Johns Hopkins University, Baltimore, MD; Northwestern University, Chicago, IL; University of Pittsburgh, Pittsburgh, PA; and David Geffen School of Medicine at UCLA and Harbor-UCLA Medical Center, Los Angeles, CA.
Abstract
Background: Although morphologic abnormalities are common among HIV-infected persons receiving highly active antiretroviral therapy (HAART), longitudinal comparative body shape changes among HAART-treated HIV-infected men versus HIV-seronegative men of similar age remain unclear.
Methods: Since September 1999, men enrolled in the Multicenter AIDS Cohort Study underwent body mass index (BMI) and circumference measurements of the waist, hip, thigh, and arm at each semiannual visit. Changes in these measurements that occurred between 1999 and 2003 among HIV-infected men were compared with measurements of HIV-seronegative men using linear mixed effects regression models. The HIV-infected men were further stratified by treatment group (no antiretroviral therapy [ART], monotherapy or combination [mono/combo] ART, or HAART). Analyses were adjusted for age, nadir CD4 cell count, and BMI (for circumference measurements).
Results: Over the 4-year observation period, mean BMI increased significantly among the 392 HIV-seronegative men (0.12 kg/m2/y; P < 0.001) but did not change in the 3 HIV-infected groups (combined n = 661). Mean waist and hip circumferences increased significantly in all groups. Hip circumferences increased more slowly in the HIV-positive HAART-treated group (n = 488) than in the HIV-seronegative group (0.18 vs. 0.49 cm/y; P < 0.001), however, yielding a more rapid increase in the waist/hip ratio in the HIV-positive, HAART-treated group over time (0.005 per year; P < 0.001).
Conclusions: The increased rate of change in waist/hip ratio in HIV-infected men receiving HAART compared with HIV-seronegative men is attributable to slower increases in hip circumference rather than an increased rate of change in waist circumference. These findings underscore the importance of body fat composition changes in the peripheral compartment relative to the central compartment among HIV-infected men receiving HAART.
Since the introduction of highly active antiretroviral therapy (HAART), changes in body composition have been increasingly reported among HIV-infected patients.1-4 These changes, collectively termed lipodystrophy, are more precisely separated into 2 processes: fat wasting (lipoatrophy) and fat accumulation (lipohypertrophy).5 Lipoatrophy typically occurs in the extremities, buttocks, and face, whereas lipohypertrophy is seen in the visceral compartment of the abdomen, in breast tissue in women and less commonly in men, and, more rarely, in the dorsocervical area. Although the consequences of these fat changes on glucose homeostasis, lipid metabolism, and cardiovascular risk may be synergistic, the pathophysiologic mechanisms underlying these fat changes are likely distinct.
Various radiographic techniques have been used to assess body composition changes. Quantitative computerized tomography (CT) or magnetic resonance imaging (MRI) can provide accurate measurements of fat in subcutaneous and deep compartments but are too expensive and cumbersome to be used routinely for clinical purposes. Dual x-ray absorptiometry (DXA) is also useful for measurement of regional fat distribution but cannot distinguish between subcutaneous and visceral fat and is not readily applicable to the general clinic population. Anthropometric measurements, such as waist, hip, and extremity circumference measurements, have the advantage of being inexpensive, and therefore readily applied in the clinic setting. In addition, some body circumference measurements, such as the waist circumference or waist/hip ratio, are important predictors of cardiovascular disease and diabetes in the general population.6-8
In studies of HIV-infected persons, those with body fat alterations discerned by clinical assessment generally have higher waist/hip ratios, marked by an increased waist circumference and decreased hip circumference, compared with those without body habitus changes.9 A similar pattern is seen when HIV-infected subjects with fat changes are compared with HIV-seronegative controls.10 When we compared HIV-infected subjects not selected on the basis of clinically apparent fat abnormalities with HIV-seronegative controls in our cohort, however, we observed similar waist/hip ratios but decreased extremity circumferences.11 This was consistent with a predominance of peripheral lipoatrophy rather than central lipohypertrophy.
Data profiling longitudinal changes in body composition are beginning to emerge and generally confirm the importance of peripheral lipoatrophy in HAART-treated patients. In a small sample of antiretroviral therapy (ART) treatment-naive men, HAART initiation was associated with a steady decrease in limb fat, but the average amount of central fat remained stable over nearly 2 years of observation.12 This study did not include an HIV-seronegative control group, however, which is a comparison that is useful to distinguish the effects of HIV and its therapy and the impact of aging.
To address the confounding effects of aging, Tien et al13 compared the incidence of confirmed self-reported lipoatrophy and central lipohypertrophy in HIV-infected women and HIV-seronegative controls in the Women's Interagency HIV Study (WIHS). Whereas the risk of peripheral lipoatrophy was more than 2 times greater in HIV-infected women, the incidence of central hypertrophy was identical between the groups. Changes in anthropomorphic measures were not reported.
The extent to which body circumference measurements change over time in HIV-infected patients and whether this differs from the trajectory of HIV-seronegative persons remain unknown. The purpose of our current study was to compare longitudinal changes in anthropomorphic measurements among HIV-infected men and HIV-seronegative control subjects. To this end, we hoped to define body composition changes that occur over time in HIV-infected patients better by using measures readily available to the clinician.
METHODS
Study Population
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective study of the natural and treated history of HIV infection among homosexual and bisexual men in the United States.14 In 1983 to 1984 and 1987 to 1991, the MACS enrolled 5622 men at 4 study sites located in Baltimore, Chicago, Pittsburgh, and Los Angeles. After an initial evaluation, MACS participants attend follow-up study visits semiannually, which consist of a physical examination, laboratory testing, and detailed interview on the use of ART in the preceding 6 months.
Beginning at the 31st MACS visit, which occurred between April and September 1999, participants underwent circumference measurements of the waist, hip, thigh, and midarm at each of the semiannual visits. Also at each visit, height and weight were used to calculate body mass index (BMI; weight in kilograms divided by height in meter squared). We restricted these analyses to MACS participants who participated in the 31st semiannual visit and reported the information regarding ART used in the previous 6 months. Of the 5622 subjects enrolled in the MACS by 1991, 1857 HIV-seronegative men were administratively censored in 1996 and 1750 men had died by April 1, 1999, leaving 2015 individuals. Of these, 1064 (53%) participated in the 31st semiannual visit. With the exclusion of 11 men who did not report the information on ART, the final study population comprised 1053 MACS participants. These analyses were based on a total of up to 4 years of follow-up from the 31st semiannual visit to the 39th semiannual visit, which occurred between April and September 2003.
Anthropomorphic Measures
A wall-mounted stadiometer was used to measure height. The participant was weighed in minimal clothing or in an examination gown. All circumferences were measured with the participant in a standing position using the protocol established in the third National Health and Nutrition Examination Survey (NHANES III).15 To minimize variability in measurement technique, examiners at the 4 sites underwent the same videotape instruction.
Assessment of Antiretroviral Therapy Exposure
HAART was defined according to the US Department of Health and Human Services (DHHS) Kaiser Panel guidelines16 as (1) 2 or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at lease 1 protease inhibitor (PI) or 1 nonnucleoside reverse transcriptase inhibitor (NNRTI), (2) 1 NRTI in combination with at least 1 PI and at least 1 NNRTI, (3) a regimen containing ritonavir and saquinavir in combination with 1 NRTI and no NNRTIs; and (4) an abacavir- or tenofovir-containing regimen of 3 or more NRTIs in the absence of PIs and NNRTIs. Combinations of zidovudine (AZT) and stavudine (d4T) with a PI or an NNRTI were not considered HAART. All combined ART regiments other than HAART were classified as combination therapy (combo-ART).
Each semiannual MACS visit includes the administration of detailed questionnaires to ascertain specific ART use during the prior 6 months. Based on this self-reported ART use, the study population was divided into 4 groups: (1) HIV-seronegative, (2) HIV-infected but not receiving ART, (3) HIV-infected receiving mono/combo-ART, and (4) HIV-infected receiving HAART. We evaluated the mono-ART group and combo-ART group together, because the mono-ART group had few person-visits (n = 38) in our study population during the study interval.
Statistical Analysis
To assess the effects of ART on body shape changes over time, multivariate linear mixed effects regression models were implemented using the SAS PROC MIXED procedure (SAS Institute, Cary, NC).17 Dependent variables were the anthropomorphic measures. Independent variables included ART exposure and a single continuous covariate representing time in years since the index visit. We specified HIV serostatus and ART exposure as a 4-level categorical variable defined previously, where the HIV-seronegative men were the reference group and this variable reflected the HIV and ART status in the prior 6 months. For adjustment, age (centered at 45 years) and nadir CD4 cell count (the lowest documented CD4 cell count at or before the baseline visit, centered at 265 cells/mm3) ascertained at the index visit were used as covariates for all models. For HIV-seronegative men, we did not include the nadir CD4 cell count in the models. BMI (centered at 25 kg/m2) was a covariate for all anthropometric measures, except for itself.
These models were fitted to the data with random effects of intercept reflecting individual differences at baseline (visit 31) and random slope of time reflecting individual rate of changes, unstructured covariance of random coefficients, and constant error variances. The restricted maximum likelihood method was used for the estimation of regression coefficients. These models provide estimates of the average linear trajectories over time while accounting for correlation among repeated measurements from the same participants. For the between-group comparisons of each outcome measure, Bonferroni correction for multiple pairwise comparisons among the 4 groups was implemented to control the family-wise error rate. A P value < 0.01 (0.05/6) was considered statistically significant for these comparisons.
The person-visits that were missing any outcome or covariate were excluded from the multiple regression models. After this exclusion, 942 participants (who contributed 6599 person-visits), 936 participants (who contributed 6533 person-visits), 935 participants (who contributed 6525 person-visits), 935 participants (who contributed 6525 person-visits), 937 participants (who contributed 6543 person-visits), and 936 participants (who contributed 6522 person-visits) were included in the models of BMI, waist circumference, hip circumference, waist/hip ratio, arm circumference, and thigh circumference, respectively.
RESULTS
Baseline Characteristics
The baseline characteristics for each of the 4 groups are shown in Table 1. The HIV-seronegative group was older and had a higher BMI than each of the HIV-infected groups. The proportion of subjects who were white was similar in the HIV-seronegative and HIV-infected mono/combo-ART group and HAART-treated group but was lower in the HIV-infected untreated group (P = 0.02 vs. HIV-infected HAART-treated group). Among the HIV-infected groups, the baseline CD4 cell count was similar. The HIV viral load was highest among untreated HIV-infected men, however. The nadir CD4 cell count was also highest in the HIV-infected no-ART group, significantly higher than in the HIV-infected mono/combo-ART group (P < 0.01) and the HIV-infected HAART-treated group (P < 0.01). The proportion of men with an undetectable viral load was higher in the HIV-infected HAART-treated group than in the HIV no-ART group (P < 0.01) and the HIV mono/combo-ART group (P < 0.01).
Baseline Anthropometric Measurements
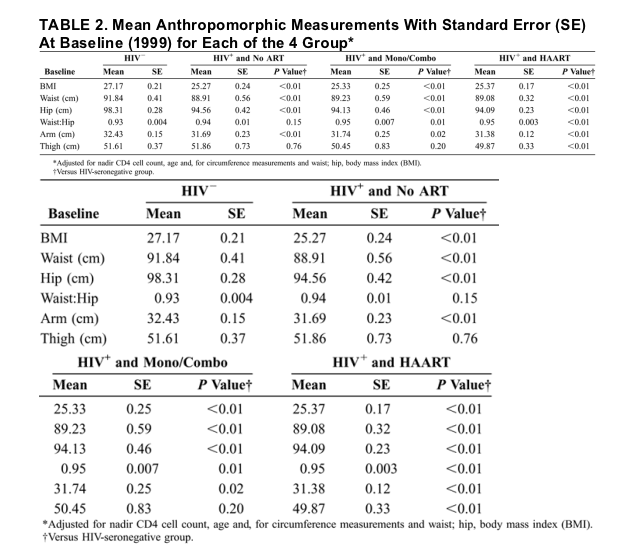
Table 2 shows mean anthropomorphic measurements for each of the 4 groups after adjustment for age, nadir CD4 cell count, and, for the body circumference measurements, baseline BMI. Adjusted waist and hip circumferences in each of the HIV-infected groups were significantly lower than in the HIV-seronegative group. Because the differences in hip circumference were proportionally larger than the differences in waist circumferences in the HIV-infected men compared with the HIV-seronegative men, the waist/hip ratios were higher in the HIV-infected groups, however. The differences reached statistical significance in the mono/combo-ART and HAART-treated groups.
Adjusted arm circumferences were lower in each of the HIV-infected groups compared with the HIV-seronegative men. Thigh circumferences were similar between the HIV-infected groups who were untreated or receiving mono- or combotherapy compared with the HIV-seronegative group but were significantly lower in the HIV-infected HAART-treated group compared with the HIV-seronegative group. When the analyses of the BMI and adjusted body circumferences were restricted to the HIV-infected groups (data not shown), only thigh circumference in the HIV-infected untreated group was significantly different than in the HIV-infected HAART-treated group (51.9 vs. 49.9 cm; P = 0.01). Other comparisons were not statistically significant.
Changes in Anthropometric Measurements Over Time
Figure 1 shows average BMI changes in each of the 4 groups over the 4-year study interval. BMI increased significantly in the HIV-seronegative group (0.12 ± 0.04 kg/m2/y; P = 0.001 vs. no change) but remained constant in each of the HIV-infected groups. Comparative differences in mean annual BMI changes were evident for the HIV-infected HAART-treated group versus the HIV-seronegative group (0.12 ± 0.04 kg/m2/y vs. -0.006 ± 0.03 kg/m2/y; P < 0.01).
Figure 2 shows the average change in body circumference measurements over the 4-year interval in each of the 4 groups after adjustment for nadir CD4 cell count, age, and BMI. Despite baseline differences, all groups showed significant yet similar increases in waist circumference. Each of the 4 groups showed significant increases in hip circumference over the study interval. The average rate of change in the HIV-infected HAART-treated group was less than in the HIV-seronegative group (0.18 ± 0.06 cm/y vs. 0.49 ± 0.07 cm/y; P < 0.01), however. When comparisons were made between the HIV-infected participants, men not treated with HAART tended to have higher rates of change in hip circumference compared with those who received HAART (HIV-infected no-ART group, 0.51 ± 0.14 cm/y, and HIV-infected monocombo-ART group, 0.63 ± 0.18 cm/y; both P = 0.03 vs. HIV-infected HAART-treated group). We observed waist/hip ratio increases over time among HAART recipients and the HIV-infected no-ART group (P = 0.027 and P = 0.001, respectively) but not in the HIV-infected mono/combo-ART group or among HIV-seronegative men. When we examined differences between treatment groups, the rate of change in the waist/hip ratio was significantly higher in the HIV-infected HAART-treated group compared with the HIV-seronegative group (0.005 ± 0.001 vs. 0.0003 ± 0.001; P < 0.01), largely reflecting differences in the rate of change in the hip circumference.
Figure 2.
Annual Change in Waist Size in first panel on the left.
The first column is HIV-negatives, the 2nd is HIV+ not ART, the 3rd is HIIV+ mono/combo ART, & the 3rd is HIV+ on HAART. You can see all groups had increases in waist size.
Annual Change in Hip Size in 2nd panel from the left.
You can see increases in hip size for all groups except HIV+ on HAART. So waist size & hip size increased in HIV-negatives, but hip size did not increase in HIV+ on HAART.
Annual Change in Waist:Hip Ratio.
This did not change in HIV-negatives but increased in HIV+ on HAART probably because there was no increase in hip size in HIV+ on HAART wwhile hip increased in HIV-negatives.
Annual change in Thigh Size.
Thigh size increased in HIV-negatives but decreased in HIV+ on HAART
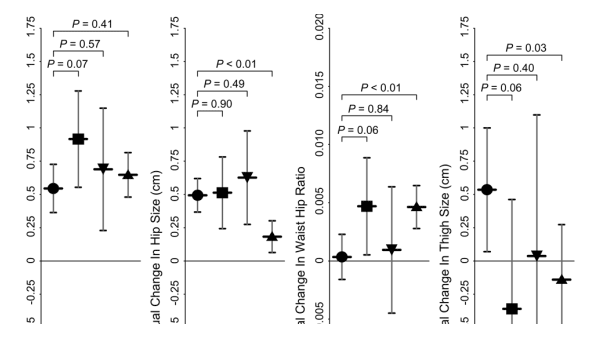
Average thigh circumference increased significantly in the HIV-seronegative groups (P = 0.024 vs. no change) but not in the HIV-infected groups. Rates of thigh circumference change in the HAART-treated group were lower than in the HIV-seronegative group (-0.14 ± 0.21 cm/y vs. 0.54 ± 0.24 cm/y; P = 0.03). Arm circumference remained constant in all the groups (data not shown).
DISCUSSION
Over 4 years of evaluation, we found that waist and hip circumferences increased significantly in all men, regardless of HIV serostatus or ART use. Although waist circumference increased at similar rates in all groups, hip circumference increased more slowly among HAART recipients compared with the HIV-seronegative men. This difference in the rate of change in hip circumference over the study interval accounted for the increased rate of change in waist/hip ratios in HAART-treated HIV-infected men compared with HIV-seronegative controls.
Waist circumference and waist/hip ratio have been used widely to assess central adiposity in the general population and are important predictors of cardiovascular disease and diabetes mellitus.6-8 Although less accurate than radiographic techniques, such as MRI or CT, that can distinguish between visceral and subcutaneous fat, these measures are inexpensive, simple to administer, and provide objective assessments of longitudinal changes in body composition.
Increases in waist circumference have been observed among HIV-infected patients in the HAART era and are related to increases in visceral fat.18 Hadigan et al19 showed that HIV-infected patients with clinically defined body fat changes had significantly greater waist circumferences than age-, gender-, and BMI-matched controls in the Framingham Offspring Cohort (94.8 vs. 91 cm; P = 0.01). These differences were seen primarily among women, however. Waist circumferences in HIV-infected men were no different than in matched controls. Similarly, a previous cross-sectional analysis from our cohort showed that compared with HIV-seronegative control subjects, waist circumferences were smaller in HIV-infected men receiving HAART not selected for body fat changes.11
To our knowledge, this is the first report of longitudinal changes in waist circumferences among HIV-infected patients. Despite baseline differences, waist circumference increased at the same rate in all the groups, irrespective of HIV status or ART received. Therefore, longitudinal assessment of waist circumference cannot distinguish HIV-infected men receiving HAART from other groups, suggesting that, on average, increases in abdominal girth are not quantitatively different in HIV-infected men compared with HIV-seronegative controls. This finding is consistent with observations from the WIHS cohort, in which HIV-infected women reported central fat accumulation over time with the same frequency as HIV-seronegative women.13
Waist/hip ratio is a complex composite measurement of regional adiposity,20,21 the biologic interpretation of which can be difficult because changes in the ratio can be affected by changes in waist or hip circumference.22 In a case-control study of HIV-infected patients with body fat abnormalities, the average waist/hip ratio was significantly higher in HIV-infected men compared with matched HIV-negative controls from the Framingham cohort. These differences were largely driven by differences in hip circumference, however.19 Extending these observations to the examination of longitudinal changes, we likewise found in the current study that differences in waist/hip ratio rates of change for HIV-infected HAART-treated subjects versus HIV-seronegative controls could be accounted for by differences hip circumference rates of change rather than by increases in rates of change for waist circumference.
Despite its limitations and complicated statistical properties,23 the waist/hip ratio may still be useful in HIV-infected patients. It is a powerful predictor of insulin sensitivity among HIV-infected patients,24 which could be attributable to the independent effects of lipoatrophy and lipohypertrophy on glucose metabolism. In addition, in the case definition proposed by Carr et al,9 waist/hip ratio was a significant predictor of lipodystrophy, independent of body fat measurements assessed by CT and MRI as well as by other demographic and laboratory variables. Nevertheless, given the similarities in the waist circumference changes seen in HIV-infected subjects and HIV-seronegative controls, at least among men, the importance of the waist/hip ratio in HIV-infected patients with lipodystrophy may be as a surrogate marker of peripheral fat wasting rather than central adiposity.
Two other findings in our study deserve mention. First, although the average change in thigh circumference was clearly different for HAART recipients compared with HIV-seronegative men, consistent with comparative patterns observed in hip circumferences, the changes in arm circumferences were no different among the HIV-infected and seronegative groups. These findings may indicate that peripheral fat wasting is more pronounced in the lower extremities. Because circumference measurements cannot distinguish between fat, muscle, and other tissues, however, this hypothesis must be pursued with more sophisticated measurement techniques.
We also found that although BMI increased progressively in the HIV-seronegative group over the study interval, it remained constant in all the HIV-infected groups. This finding was also seen among women in the WIHS cohort.13 Although the factors contributing to this observation are not clear, the long-term effects of HIV infection itself and/or its therapy are the most appealing explanations. In our cohort, the lack of change in BMI in all HIV-infected patients, regardless of treatment group, would suggest that HIV infection plays an important role in preventing the weight gain observed in HIV-seronegative men. Other differences among the groups, however, such as diet and exercise patterns, could also contribute to this finding.
To distinguish the role of HIV infection and HIV therapy on these anthropomorphic measurements better, we included 2 other groups of HIV-infected men: those not receiving HAART and those not receiving any therapy. This analysis was limited by the small size of these groups and the fact that they might have had prior exposure to other antiretrovirals before the time period included in this analysis. Classification by ART was updated every 6 months, but the effects of prior regimens may have been persistent. As a result, it is difficult to draw conclusions based on the data of these groups.
Our study has additional limitations. Anthropomorphic measurements were obtained in the MACS beginning in 1999, 3 years after the introduction of HAART. Because we do not have data for HAART recipients immediately subsequent to therapy initiation, we cannot exclude other body habitus changes in the time after HAART initiation. Mallon et al25 observed increases in peripheral and central fat immediately after HAART initiation, followed by stabilization in central fat at week 24 and progressive loss of peripheral fat. Our findings complement this observation by providing a window on body shape changes several years after HAART initiation. Second, our study used only anthropomorphic measurements to assess body composition. More sophisticated techniques, such as DXA, MRI, or CT, would have provided a clearer view of relative changes in body tissue composition over time. Furthermore, because the MACS consists only of men, it is unclear whether these findings can be generalized to women. Consistent with our results, however, the longitudinal assessment of self-reported body composition in the WIHS demonstrated that central fat accumulated in women similarly regardless of HIV serostatus.13 Finally, in this analysis, we did not investigate which components of HAART are associated with anthropometric changes. This is a subject of ongoing investigation in our cohort.
In conclusion, we found that HIV-infected men receiving HAART had similar rates of increase in waist circumference as HIV-seronegative men over the 4-year interval. In addition, the more rapid changes in waist/hip ratios observed among HIV-infected patients receiving HAART were attributable to differences in rates of change in hip circumference. These findings underscore the fact that peripheral fat wasting is the more predominant body composition alteration among HIV-infected patients receiving HAART and that changes in waist/hip ratio do not necessarily represent changes in central adiposity in this population.
REFERENCES
1. Carr A, Samaras K, Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093-2099.
[Medline Link] [CrossRef] [Context Link]
2. Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389-1398.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
3. Lichtenstein KA, Delaney KM, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr. 2003;32:48-56.
[Fulltext Link] [Medline Link] [Context Link]
4. Shikuma CM, Waslien C, McKeague J, et al. Fasting hyperinsulinemia and increased waist-to-hip ratios in non-wasting individuals with AIDS. AIDS. 1999;13:1359-1365.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
5. Lichtenstein KA. Redefining lipodystrophy syndrome: risks and impact on clinical decision making. J Acquir Immune Defic Syndr. 2005;39:395-400.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
6. Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555-563.
[Medline Link] [Context Link]
7. Larsson B, Svardsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. BMJ. 1984;288:1401-1404.
[Context Link]
8. Seidell JC, Han TS, Feskens EJ, et al. Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med. 1997;242:401-406.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
9. Carr A, Emery S, Law M, et al. An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet. 2003;361:726-735.
[Medline Link] [CrossRef] [Context Link]
10. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130-139.
[Medline Link] [CrossRef] [Context Link]
11. Palella FJ Jr, Cole SR, Chmiel JS, et al. Anthropometrics and examiner-reported body habitus abnormalities in the multicenter AIDS cohort study. Clin Infect Dis. 2004;38:903-907.
[Medline Link] [CrossRef] [Context Link]
12. Mallon PW, Miller J, Cooper DA, et al. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971-979.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
13. Tien PC, Cole SR, Williams CM, et al. Incidence of lipoatrophy and lipohypertrophy in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2003;34:461-466.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
14. Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310-318.
[Medline Link] [Context Link]
15. National Health and Nutrition Examination Survey III. Body Measurements (Anthropometry). Westat, Rockville, MD; 1988.
[Context Link]
16. Dybul M, Fauci AS, Bartlett JG, et al. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137:381-433.
[Medline Link] [Context Link]
17. Littell RC, Milliken GA, Stroup WW, et al. SAS System for Mixed Models. SAS Press, Cary, NC; 1996.
[Context Link]
18. Miller KD, Jones E, Yanovski JA, et al. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871-875.
[Medline Link] [CrossRef] [Context Link]
19. Hadigan C, Meigs JB, Wilson PW, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909-916.
[Medline Link] [CrossRef] [Context Link]
20. Krotkiewski M, Bjorntorp P, Sjostrom L, et al. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150-1162.
[Medline Link] [Context Link]
21. Hartz AJ, Rupley DC Jr, Kalkhoff RD, et al. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev Med. 1983;12:351-357.
[Medline Link] [CrossRef] [Context Link]
22. Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness-a critical review. Int J Obes Relat Metab Disord. 1998;22:719-727.
[Context Link]
23. Allison DB, Paultre F, Goran MI, et al. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995;19:644-652.
[Context Link]
24. Meininger G, Hadigan C, Rietschel P, et al. Body-composition measurements as predictors of glucose and insulin abnormalities in HIV-positive men. Am J Clin Nutr. 2002;76:460-465.
[Medline Link] [Context Link]
25. Mallon PW, Miller J, Cooper DA, et al. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971-979.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
|
|
| |
| |
|
|
|