| |
Pravastain + Exetimibe for LDL
|
| |
| |
Ezetimibe, a promising lipid-lowering agent for the treatment of dyslipidaemia in HIV-infected patients with poor response to statins
AIDS: Volume 20(17) 14 November 2006 p 2159-2164
Negredo, Eugeniaa,b; Molto, Josea; Puig, Jordia; Cinquegrana, Denisea; Bonjoch, Annaa; Perez-Alvarez, Nuriaa; Lopez-Blazquez, Raquela; Blanco, Asuncionc; Clotet, Bonaventuraa,c; Rey-Joly, Celestinob
From the aLluita contra la SIDA, Germans Trias i Pujol Hospital, Universitat Autonoma de Barcelona, Barcelona, Spain
bInternal Medicine Department, Germans Trias i Pujol Hospital, Universitat Autonoma de Barcelona, Barcelona, Spain
cIrsicaixa Foundation, Germans Trias i Pujol Hospital, Universitat Autonoma de Barcelona, Barcelona, Spain.
"....Studies conducted in the general population with primary hypercholesterolaemia show that the addition of ezetimibe to statin therapy provides a supplementary benefit, lowering LDL-cholesterol by an additional 25% [1-5]. In HIV-infected patients, our results show that more than 60% of the study population, who had poorly controlled dyslipidaemia while using pravastatin alone, lowered LDL-cholesterol levels to normal levels after starting ezetimibe. In addition, factors such as HDL-cholesterol and apoprotein A levels, which are inversely associated with cardiovascular risk, significantly increased, although no changes were noted in inflammatory parameters...."
Abstract
Objective: To assess the efficacy, safety, and pharmacokinetic interactions of ezetimibe in HIV-infected patients with poorly controlled antiretroviral-associated dyslipidaemia while taking pravastatin alone.
Design: A prospective, open-label, one-arm study of 24 weeks duration.
Patients and setting: Nineteen patients (18 on stable HAART), with low density lipoprotein (LDL)-cholesterol values of ≥ 130 mg/dl despite the use of pravastatin.
Methods: Ezetimibe, 10 mg/day, was added to pravastatin 20 mg/day, while patients maintained the same antiretroviral regimen. Determinations of total, LDL-, and high density lipoprotein (HDL)-cholesterol, triglycerides, apoproteins, and inflammatory factors (homocystein and C-reactive protein) were performed at baseline, and at weeks 6, 12, and 24. Liver enzymes and creatinine phosphokinase were also assessed. Protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI) Cmin was determined just before and 12 weeks after ezetimibe introduction.
Results:
At week 24, 61.5% of patients achieved the endpoint of the study (LDL-cholesterol < 130 mg/dl).
Significant declines in mean total and LDL-cholesterol levels were observed between baseline and weeks 6, 12, and 24, irrespective of antiretroviral type (PI or NNRTI).
Mean HDL-cholesterol and apoprotein A increased significantly.
No patients discontinued therapy due to intolerance or presented toxicity of grade 2 or more.
No differences were observed in lopinavir or nevirapine Cmin measured just before and 12 weeks after ezetimibe introduction.
Conclusion: The addition of ezetimibe to ongoing pravastatin seems to be an effective and safe option for HIV-infected patients not achieving the NCEP ATPIII LDL-cholesterol goals while receiving a statin alone. Its high tolerability and the lack of interactions with the cytochrome CYP3A4 indicate that ezetimibe will not increase the risk of toxicity or pharmacokinetic interactions with antiretrovirals.
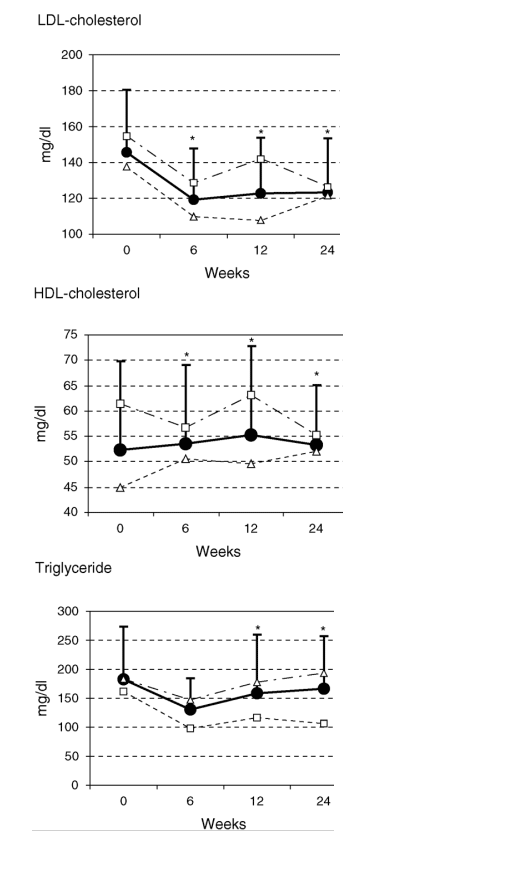
Fig. 1. Evolution of lipid parameters from baseline to week 24, considering all patients (black circles), and according to type of antiretroviral (white squares for PI and white triangles for NNRTI). (* P < 0.05 at that visit point from baseline).
Total Cholesterol
Introduction
Ezetimibe is the first selective inhibitor of cholesterol absorption at the intestinal level. It reduces cholesterol absorption in the duodenum by approximately 50%, thereby attaining reductions in low density lipoprotein (LDL)-cholesterol of 20%. This benefit is significantly greater when it is given with any of the statins, achieving reductions in LDL-cholesterol of up to 50% [1-5]. This synergistic effect of the two drugs in combination results from the inhibition of duodenal cholesterol absorption by ezetimibe, together with the reduction of hepatic cholesterol production by the statins.
In the HIV-infected population, the prevalence of antiretroviral-related dyslipidaemia is high, and is mainly associated with the use of protease inhibitors (PI) [6,7]. Furthermore, this disorder is even more frequent in patients with fat redistribution or lipodystrophy [8].
According to the US-based Adult AIDS Clinical Trial Group (AACTG) Cardiovascular Disease Focus Group, treatment with pravastatin, fluvastatin, or atorvastatin is recommended for antiretroviral-linked hypercholesterolaemia, while lovastatin and simvastatin therapy should be avoided due to interactions with PI or non-nucleoside reverse transcriptase inhibitors (NNRTI) [9] and the risk of skeletal muscle toxicity.
Nonetheless, many studies that evaluate the effect of statins for the treatment of antiretroviral-associated dyslipidaemia have shown only partial responses to such therapy, with total and LDL-cholesterol values being reduced by just 25% [10].
The addition of ezetimibe to a low-dose statin has not previously been evaluated in the HIV-infected population with antiretroviral-related dyslipidaemia. Moreover, the properties of ezetimibe, including its good tolerability and lack of interactions with the cytochrome CYP 3A4 metabolic pathway [11,12], suggest that ezetimibe will not increase the risk of toxicity or pharmacokinetic interactions with antiretrovirals. Likewise, ezetimibe has a once-a-day administration, which will not contribute a significant additional pill burden to patients' daily medication regimens. As a result, ezetimibe might be a promising lipid-lowering candidate for the treatment of hypercholesterolaemia associated with antiretrovirals, mainly in patients with a poor response to statins.
Methods
Study design, objectives, and participants
This is a prospective, one-arm, open-label, pilot study with 24 weeks of follow-up. The main objective was to assess the efficacy on lipid and inflammatory parameters of the addition of ezetimibe, 10 mg/day, to ongoing pravastatin in HIV-infected patients with poorly controlled dyslipidaemia. Secondary objectives included an evaluation of safety and pharmacokinetic interactions between ezetimibe and antiretroviral agents.
Candidates were HIV-1 infected patients with LDL-cholesterol values of ≥ 130 mg/dl within the last 3 months despite the use of pravastatin, 20 mg/day, for at least the previous 6 months. In order to be included, patients must have either maintained a stable antiretroviral regimen or had to remain free of any antiretroviral therapy for the 3 months prior to the study. In addition, in order to avoid interferences in calculating LDL-cholesterol, patients with triglyceride levels higher than 350 mg/dl were excluded from the study. Finally, only patients who signed the study's informed consent form were included in this investigation.
Follow-up and assessment
Changes in lipid metabolism and inflammatory parameters were investigated. Specifically, determinations of total, LDL-, and high density lipoprotein (HDL)-cholesterol, triglycerides, apoproteins A and B, and inflammatory factors such as homocystein and C-reactive protein (CRP) were performed at baseline, and at weeks 6, 12, and 24. Routine biochemistry and haematology were tested at the same time points, paying special attention to liver enzymes [aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltranspeptidase (GGT)] and to creatinine phosphokinase (CK).
In addition, NNRTI and PI concentrations in plasma were determined before the morning dose (minimum concentration, Cmin), at baseline and 12 weeks after the introduction of ezetimibe. Drug concentrations were assessed by HPLC following a previously validated method. Our laboratory is subjected to the KKGT quality assurance program organized by the Dutch Association for Quality Assessment in Therapeutic Drug Monitoring and Clinical Toxicology of the Radbound University Medical Centre in Nijmen, with 36 laboratories involved in 2004 [13].
All patients were recommended to maintain their usual diet and exercise regimen throughout the follow-up of the study. Thus, patients who incorporated diet or exercise changes, those who changed their antiretroviral combination, or those who changed their lipid-lowering medication, discontinued the study. Likewise, subjects who presented an adverse event of severity grade 2 or higher, particularly liver toxicity or skeletal muscle toxicity, also withdrew ezetimibe.
Study endpoints
The primary efficacy endpoint was the percentage of subjects who reduced their LDL-cholesterol levels to ≦ 130 mg/dl, at weeks 6 and 24. In addition, changes in all lipid and inflammatory parameters were equally evaluated throughout the follow-up, as well as the percentage change at week 24 with respect to baseline values in all parameters.
Safety was evaluated by determining the percentage of patients who did not tolerate and subsequently discontinued ezetimibe, in addition to determining the percentage of patients with toxicity of at least grade 2 severity (liver enzymes or CK increase grade 2 or higher).
In order to assess possible drug interactions between ezetimibe and antiretroviral agents, Cmin of PI and NNRTI were compared before and after the introduction of ezetimibe.
Results
Nineteen 19 patients were included, 13 males, 6 females. The mean (± SD) age was 43 years (± 9), and the mean BMI was 25.7 kg/m2 (± 4). Ten percent of the study subjects were smokers. All patients except one included in the study were taking HAART: 10 were receiving a PI-containing combination (eight on lopinavir/ritonavir, one on nelfinavir, and one on atazanavir/ritonavir) and eight were receiving an NNRTI-based therapy (seven on nevirapine and one on efavirenz).
At weeks 6 and 24 of the study, LDL-cholesterol levels were ≦ 130 mg/dl in 62.5% and 61.5% of patients, respectively. No differences were seen when patients were analysed according to type of antiretroviral therapy (62.5% of patients receiving a PI and 60% of patients on an NNRTI; P > 0.05).
Figure 1 shows the evolution in lipid parameters throughout the duration of the study, once again considering all patients, and according to type of antiretroviral (PI or NNRTI). Significant declines were observed for all patients between baseline values and weeks 6, 12, and 24, in total cholesterol (P = 0.014, P = 0.013, and P = 0.011, respectively) and LDL-cholesterol (P = 0.003, P = 0.048, and P = 0.005, respectively.) On the contrary, significant increases were observed in HDL-cholesterol (P < 0.001 at weeks 6 and 12, and P = 0.004 at week 24) and apoprotein A (P = 0.006 at week 12 and P = 0.007 at week 24). Triglyceride, apoprotein B, homocystein or CRP levels remained unchanged from baseline to week 24 (P = 0.209, P = 0.086, P = 0.112, and P = 0.859, respectively). No differences were observed in terms of lipid parameter evolution, according to type of antiretroviral (Fig. 1).
The tolerability of the addition of ezetimibe, 10 mg, to pravastatin 20 mg daily, was excellent in all patients. No patients discontinued therapy due to toxicity, nor presented increases in liver enzymes or CK levels of grade 2 or higher.
Three patients were excluded from the study due to a violation of the protocol: two patients changed their antiretroviral regimen (one at week 6 and one at week 12), and one patient added a fibrate during the study follow-up (at week 12). A fourth patient voluntarily abandoned the study (at week 12).
Drug concentrations before and after the introduction of ezetimibe were compared in patients on nevirapine (n = 7) or lopinavir/ritonavir therapy (n = 8). Median (range) nevirapine Cmin was 6.2 (3.6-8.2) mg/l before and 6.0 (3.6-7.2) mg/l 12 weeks after the introduction of ezetimibe (P = 0.753). Similarly, lopinavir Cmin was 7.3 (5.2-9.6) mg/l and 8.5 (6.4-17.9) mg/l at baseline and at week 12, respectively (P = 0.345).
Discussion
Many cohort studies indicate a higher incidence of cardiovascular disease in the aging HIV-infected population, associated with standard risk factors [14-16]. Consequently, the modification of these factors is necessary in these patients to prevent cardiovascular mortality, just as demonstrated in the general population. Hyperlipidaemia is one of the most common risk factors in these subjects, together with smoking, insulin resistance, or hypertension, which frequently requires intervention. However, only a small proportion of these patients actually receive adequate intervention [17].
The substitution of PI for other antiretroviral agents with a less negative impact on lipid metabolism, such as NNRTI [18-20] or atazanavir [21] is not always possible due to viral or immunological factors, which must have first priority when determining the appropriate therapy for patients. Therefore, pharmacologic therapy with lipid-lowering medications, specifically statins, needs to be used without jeopardizing the antiviral benefit of the antiretroviral regimen. This approach is likely to achieve treatment goals in 50-80% of the general population, according to the NCEP ATP III goals [22], but responses to statins in HIV-infected patients is more often partial and insufficient [10]. Pravastatin is one of the few lipid-lowering agents recommended for use in this setting, since the majority of such agents could provoke drug interactions with antiretrovirals that may result in an increased risk of drug-related toxicity [9]. In cases of partial response, while increasing the dosage of statins or adding fibrates would increase the benefit on lipid parameters, it would also raise the risk of toxicity.
Since the maintenance of cholesterol homeostasis not only depends on the de novo cholesterogenesis but also on the intestinal absorption of diet-derived cholesterol, other strategies to reduce plasma cholesterol are being investigated. The understanding of the mechanism of action of a number of transporter proteins involved in the absorption of diet-derived cholesterol in the small intestine has lead to the development of novel cholesterol-lowering strategies. One of the most recently identified transporter proteins is the Niemann-Pick C1-like1 protein, which is a key player in cholesterol absorption by the small intestine and may represent a target of the cholesterol absorption inhibitor ezetimibe [23].
Studies conducted in the general population with primary hypercholesterolaemia show that the addition of ezetimibe to statin therapy provides a supplementary benefit, lowering LDL-cholesterol by an additional 25% [1-5]. In HIV-infected patients, our results show that more than 60% of the study population, who had poorly controlled dyslipidaemia while using pravastatin alone, lowered LDL-cholesterol levels to normal levels after starting ezetimibe. In addition, factors such as HDL-cholesterol and apoprotein A levels, which are inversely associated with cardiovascular risk, significantly increased, although no changes were noted in inflammatory parameters.
Additionally, no adverse events were reported during the study, confirming the high tolerability already described in the general population. Finally, plasma nevirapine or lopinavir concentrations remained unchanged after ezetimibe introduction. Importantly, this lack of effect on NNRTI or PI concentrations suggests that while ezetimibe inhibits absorption of cholesterol at the intestinal level through a mechanism that is still not well understood, it does not seem to interfere with the absorption of antiretroviral agents. On the other hand, although it was not assessed in this study, the different metabolic pathways of ezetimibe and PI or NNRTI make no significant changes on ezetimibe concentrations in plasma to be expected during its concomitant use.
Despite the small sample size of the study, our clinical and pharmacokinetic results are encouraging. Specifically, the results seem to be sufficiently solid to consider ezetimibe as a complementary lipid-lowering drug for the treatment of antiretroviral-associated dyslipidaemia in patients with poor responses to statins.
In summary, the addition of ezetimibe to ongoing pravastatin could be an effective and safe option for HIV-infected patients not achieving the NCEP ATP III LDL-cholesterol goals while receiving a statin alone. Additionally, this strategy was not associated with increased toxicity or significant drug-drug interactions.
References
1. Masana L, Mata P, Gagne C, Sirah W, Cho M, Johnson-Levonas AO, et al. Long-term safety and, tolerability profiles and lipid-modifying efficacy of ezetimibe coadministered with ongoing simvastatin treatment: a multicenter, randomized, double-blind, placebo-controlled, 48-week extension study. Clin Ther 2005; 27:174-184.
[Medline Link] [CrossRef] [Context Link]
2. Gagne C, Bays HE, Weiss SR, Mata P, Quinto K, Melino M, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol 2002; 90:1084-1091.
[Medline Link] [Context Link]
3. Gagne C, Gaudet D, Bruckert E, Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 2002; 105:2469-2475.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
4. Kerzner B, Corbelli J, Sharp S, Lipka LJ, Melani L, LeBeaut A, et al. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol 2003; 91:418-424.
[Medline Link] [CrossRef] [Context Link]
5. Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc 2005; 80:585-586.
[Context Link]
6. Carr A, Samaras K, Thorisdottir A, Kaufmann G, Chisholm D, Cooper D. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999; 353:2093-2099.
[Medline Link] [CrossRef] [Context Link]
7. Friis-Moller N, Reiss P, El-Sadr W, D'Arminio A, Monfort R, Thiebaut S, et al. Exposure to PI and NNRTI and risk of myocardial infarction: results from the D:A:D Study. Thirteenth Conference on Retroviruses and Opportunistic Infections. Denver, CO, February 2006 [abstract 144].
[Context Link]
8. Carr A. HIV lipodystrophy: risk factors, pathogenesis, diagnosis and management. AIDS 2003; 17:S141-S148.
[Fulltext Link] [Medline Link] [Context Link]
9. Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, Blaschke T, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 2002; 16:569-577.
[Context Link]
10. Calza L, Manfredi R, Chiodo F. Statins and fibrate for the treatment of hyperlipidaemia in HIV-infected patients receiving HAART. AIDS 2003; 17:851-859.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
11. Reyderman L, Kosoglou T, Cutler DL, Maxwell S, Statkevich P. The effect of fluvastatin on the pharmacokinetics and pharmacodynamics of ezetimibe. Curr Med Res Opin 2005; 21:1171-1179.
[Medline Link] [CrossRef] [Context Link]
12. Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet 2005; 44:467-494.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
13. Droste JAH, Aarnoutse RE, Koopmans PP, Hekster YA, Burger DM. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J Acquir Immune Defic Syndr 2003; 32:287-291.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
14. Lichtenstein K, Armon C, Buchacz K, Moorman A, Wood K, Brooks J, et al. Analysis of cardiovascular risk factors in the HIV Outpatient Study Cohort. Thirteenth Conference on Retroviruses and Opportunistic Infections. Denver, CO, February 2006 [abstract 735].
[Context Link]
15. Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D, Clinical Epidemiology Group from the French Hospital Database. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS 2003; 17:2529-2531.
[Context Link]
16. Friis-Moller N, Sabin CA, Weber R, d'Armonio Monforte A, El-Sadr WM, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993-2003.
[Medline Link] [CrossRef] [Context Link]
17. Bucher H, Glass T, Weber R. and the Swiss HIV Cohort Study. Predictors of successful blood pressure management in HIV-infected patients at moderate to high 10-year risk of coronary heart disease: The Swiss HIV Cohort Study. Thirteenth Conference on Retroviruses and Opportunistic Infections. Denver, CO, February 2006 [abstract 740].
[Context Link]
18. Negredo E, Ribalta J, Paredes R, Ferre R, Sirera G, Ruiz L, et al. Reversal of atherogenic lipoprotein profile in HIV-1 infected patients with lipodystrophy after replacing protease inhibitors by nevirapine. AIDS 2002; 16:1383-1389.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
19. Martinez E, Arnaiz JA, Podzamczer D, Dalmau D, Ribera E, Domingo P, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. New Engl J Med 2003; 349:1036-1046.
[Medline Link] [CrossRef] [Context Link]
20. Raffi F, Bonnet B, Ferre V, Esnault JL, Perre P, Reliquet V, et al. Substitution of a Nonnucleoside Reverse transcriptase inhibitor for a Protease Inhibitor in the treatment of Patient with undetectable plasma human immunodeficiency virus type 1 RNA. Clin Infect Dis 2000; 31:1274-1278.
[Medline Link] [CrossRef] [Context Link]
21. Gatell J, Salmon-Ceron D, Lazzarin A, Van Wijngaerden E, Antunes F, Leen C, et al. Efficacy and safety of Atazanavir (ATV) based HAART in patients switched from a stable boosted/ unboosted protease inhibitor (PI) treatment. The Swan study. Tenth European AIDS Society/ EACS. Dublin, Ireland, November 2005 [abstract PS1/1].
[Context Link]
22. McKenney JM. Pharmacologic options for aggressive low-density lipoprotein cholesterol lowering: benefits versus risks. Am J Cardiol 2005; 96:60E-66E.
[Medline Link] [Context Link]
23. Plosch T, Kosters A, Groen AK, Kuipers F. The ABC of hepatic and intestinal cholesterol transport. Handb Exp Pharmacol 2005; 170:465-482.
[Context Link]
|
|
| |
| |
|
|
|