| |
Does rHGH Help or Harm Body Composition?
|
| |
| |
Reported by Jules Levin
Below are two study reports. The first was published in the journal HIV Medicine and reports 24 week response of rHGH 4mg daily or 3 times a week for 12 weeks, followed by a 2 mg daily maintenance dose for 12 weeks. The second report was a poster at ICAAV Sept 2006. This dosing scheme was similar to that used in the study reported at the IAS Toronto meeting as a Late Breaker oral, the data from which I think will be used as a pivotal study for FDA approval for treatment of lipodystrophy or what Serono calls HARS (HIV-associated Adipose Redistribution Syndrome). From my interpretation of the data, from the ICAAC poster you will see VAT (belly fat) increases during the 9-12 month followup after stopping rHGH. As well, you will see that at week 24 on treatment there is facial fat loss and fat loss in other areas. After rHGH is stopped there is a little increase during the followup period. In sum, these data suggest to me that if one discontinues rHGH belly fat returns; and while on rHGH facial fat & lipoatrophy in other body areas worsen. My understanding is that the FDA will be holding a public hearing for approval pf rHGH with an indication for improved body composition.
A randomized, open-label study to compare the effects of two different doses of recombinant human growth hormone on fat reduction and fasting metabolic parameters in HIV-1-infected patients with lipodystrophy
HIV Medicine
Volume 7 Page 397 - September 2006
M Bickel,1 S Zangos,2 V Jacobi,2 T Lutz,3 G Knecht,5 F Goebel,4 S Staszewski1 and S Klauke5
1HIV Treatment and Research Unit and 2Department of Radiology, JW Goethe University, Frankfurt, Germany,
3Gemeinschaftspraxis Grueneburgweg, Frankfurt, 4Medizinische Poliklinik, Klinikum der LMU Munich, Munich, Germany,
and 5Internistisches Facharzt Zentrum Stresemannalle (IFS), Frankfurt, Germany
.....in Introduction Author says: "...the optimal dose and duration of r-hGH treatment are yet to be determined. Concerns about a reduction of subcutaneous fat, especially in the facial region, and therefore a perceived worsening of fat loss were raised....." (and in Conclusion says: "...this exploratory pilot study showed that subcutaneous facial and thigh peripheral fat reductions following 12 weeks of treatment did not differ between patients on two different doses of r-hGH, and no further reduction was observed during maintenance therapy...."
Background
Several studies have shown beneficial effects of recombinant human growth hormone (r-hGH) in reducing visceral adipose tissue (VAT) in HIV-1-infected patients with lipodystrophy.
Methods
The 24041 trial was a randomized, open-label pilot study conducted at one private practice and two university hospitals in Germany.
Patients were randomized to r-hGH 4mg daily (group A) or three times per week (group B) over 12 weeks, followed by a 2 mg daily maintenance dose for 12 weeks. Magnetic resonance imaging (MRI) scans were performed to assess body composition.
Exclusion criteria were active opportunistic infection, malignancy (cutaneous Kaposi's sarcoma with up to five lesions was allowed), uncontrolled hypertension, diabetes mellitus and carpal tunnel syndrome. The primary endpoint was change of visceral adipose tissue (VAT). Secondary endpoints were changes of subcutaneous fat in the face, legs and abdomen, metabolic parameters and body composition as assessed by anthropometric measurements.
Results
A total of 26 subjects were included in the study. VAT was reduced overall by 35.1 cm2 (29.5%) at week 12 and by 49 cm2 (39.9%) at week 24, respectively, compared with baseline (Po0.001 for both comparisons).
By week 12, VAT was reduced by 27 and 29% (A vs B; P50.47) while facial fat was reduced by 3.3 and 2.6 cm2 in groups A and B, respectively (P50.96).
Over 24 weeks, VAT was reduced by 42 and 38% (P50.35) and facial fat by 3.2 and 2.4 cm2 in groups A and B, respectively (P50.91), compared with baseline.
There was a greater increase in high-density lipoprotein (HDL) in group A than in group B (4.9 vs 2.4 mg/dL in week 12 and 7.1 vs 0.4 mg/dL in week 24; P50.03).
Fasting insulin levels increased, whereas glucose and insulin measured in oral glucose tolerance tests remained unchanged. Drug-related side effects were transient and reversible, but more common in group A (67%) than in group B (29%).
Conclusions
This study confirms reports that r-hGH effectively reduces VAT, with a relatively small reduction of facial and limb fat.
Subjects and safety
Twenty-six patients (25 male and one female; mean age 44.8 years) were included in the study. Twelve patients were randomized to receive 4 mg of r-hGH daily (group A) and 14 patients received 4 mg r-hGH three times per week (group B). Four patients (three in group A) permanently stopped the study drug before week 12. Two of these four patients experienced a serious AE. One 50-year-old male patient tried to commit suicide. The second patient, a 60-year-old man, was hospitalized because of creatinine kinase (CK) elevation; silent myocardial infarction was suspected but could be ruled out; r-hGH was stopped and he recovered completely. Both events were assessed by the investigator as being not related to the study drug. The other two patients withdrew consent because of nonserious AEs (loss of appetite and diarrhoea in one case, and myalgia and athralgia in the other), which also resolved completely after stopping the study drug. For three patients (all in group A), the dose of the study drug was reduced until week 12, as defined in the study protocol, because of nonserious AEs (transient hyperglycaemia, muscle pain and peripheral oedema). The AEs resolved and all three patients completed the 24-week study period. During the maintenance phase, between weeks 12 and 24, two more patients (one from each group) discontinued because of AEs (myalgia and oedema in one case, and athralgia and insomnia in the other), both without prior dose reduction. Most of the common side effects related to the study drug, such as peripheral oedema, myalgia, arthralgia and dysesthesia, were mild and transient. Overall, AEs probably related to r-hGH were more often reported in group A (67% of subjects) than in group B (29%). Four patients in group A experienced severe side effects possibly related to the study drug (dyspepsia, peripheral oedema, transient hyperglycaemia and pain in the extremities), whereas none was reported for group B. The dose of r-hGH in the patient with the hyperglycaemia was reduced and the AE resolved thereafter.
Fat distribution and body composition
Baseline values and changes in primary and secondary endpoints are summarized in Table 2, see Tables below. VAT was reduced overall by 35.1 cm2 (29.5%) at week 12 and by 49 cm2 (39.9%) at week 24 compared with baseline (P<0.001 for both comparisons). At baseline, VAT was significantly higher in patients in group B than in those in group A. There was no statistically significant difference between groups A and B in reduction of VAT, either for the randomization [28.3 cm2 for group A vs 39.9 cm2 for group B; P=0.47; nonsignificant dose comparison (NSDC)] or for the maintenance phase (43.1 cm2 for group A vs 52.7 cm2 for group B; P=0.35; NSDC). At week 12, SAT was reduced by 13.3% in group A and by 12.6% in group B (P=0.97; NSDC). During the maintenance phase, SAT was further decreased by 3.3% in group A and by 0% in group B (P=0.99; NSDC) between weeks 12 and 24. Accordingly, the overall VAT/TAT ratio decreased significantly from baseline to week 12 (0.40 vs 0.36, respectively; P=0.01) and between weeks 12 and 24 (0.36 vs 0.34, respectively; P<0.001) with no significant difference between groups (NSDC).
Subcutaneous mid-thigh fat in the right leg was similarly reduced at week 12 (14.2% for group A vs 12.7% for group B; P=0.82; NSDC). During the maintenance phase, mid-thigh fat was further decreased by 6.9% in group A but increased slightly by 2.8% in group B (P=0.33; NSDC), while mid-thigh volume did not change in either group. Facial fat was reduced overall by 19.7% at week 12 (20.1% for group A vs 19.1% for group B; P=0.96; NSDC), but increased slightly during the maintenance phase (0.6 for group A vs 1.5% for group B; P=0.94; NSDC). Waist and hip circumferences decreased significantly at weeks 12 and 24 compared with baseline for all patients (P<0.05 for all changes). This reduction was more pronounced for patients in group A than for those in group B, although the difference was not statistically significant. In contrast, the leg circumference remained unchanged during the study. Body weight increased slightly in both groups until week 12 (0.7 kg for group A vs 0.9 kg for group B; P=0.78). After 24 weeks it had decreased in group A by 0.7 kg and increased by 1.7 kg in group B compared with baseline (P=0.27). Total body fat assessed by BIA was reduced to a significantly greater extent in group A than in group B at week 12 [7.4 kg in group A vs 3.6 kg in group B; P=0.046; significant dose comparison (SDC)]. During the maintenance phase, body fat increased slightly in group A, but decreased in group B (0.8 kg in group A vs -0.9 kg in group B; P=0.35); the difference compared with baseline was nonsignificant (P=0.36). In contrast, body cell mass (BCM) showed a greater increase in group A than in group B at weeks 12 and 24 compared with baseline, but the difference was again not significant (week 12, 3.4 kg for group A vs 1.2 kg for group B; P=0.35; week 24, 2.5 kg for group A vs 2.0 kg for group B; P=0.93).
Metabolic parameters
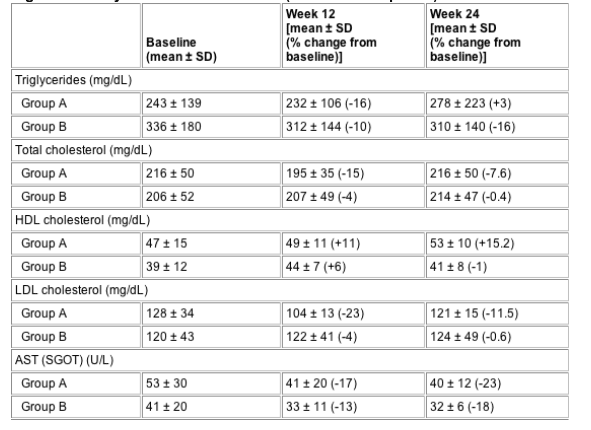
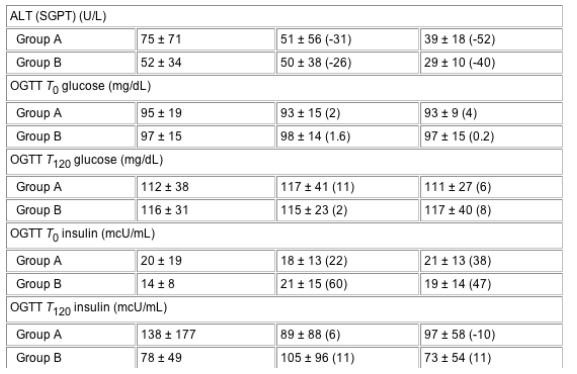
Total cholesterol was reduced by 31.3 and 8.1 mg/dL over 12 weeks in groups A and B, respectively (P=0.046; SDC) and maintained over 24 weeks. There was a greater reduction in LDL in group A than in group B (28.9 vs 4.2 mg/dL at week 12 and 14.7 vs 0.7 mg/dL at week 24, respectively; NSDC). There was a significantly greater increase in HDL in group A than in group B after 24 weeks (4.9 vs 2.4 mg/dL at week 12 and 7.1 mg/dL vs -0.4 mg/dL at week 24, respectively; P=0.03; SDC). Overall, fasting insulin and c-peptide increased significantly at weeks 12 and 24, respectively (NSDC). Fasting glucose, insulin and c-peptide levels during the OGTT did not change either compared with baseline or between the two groups (Table 3, see Table below).
Table 2 Evolution of anthropometric and metabolic parameters for patients treated with 4 mg r-hGH daily (group A), compared with those treated with 4 mg r-hGH three times per week (group B) for 12 weeks (randomization phase) followed by 2 mg r-hGH daily for another 12 weeks (maintenance phase)
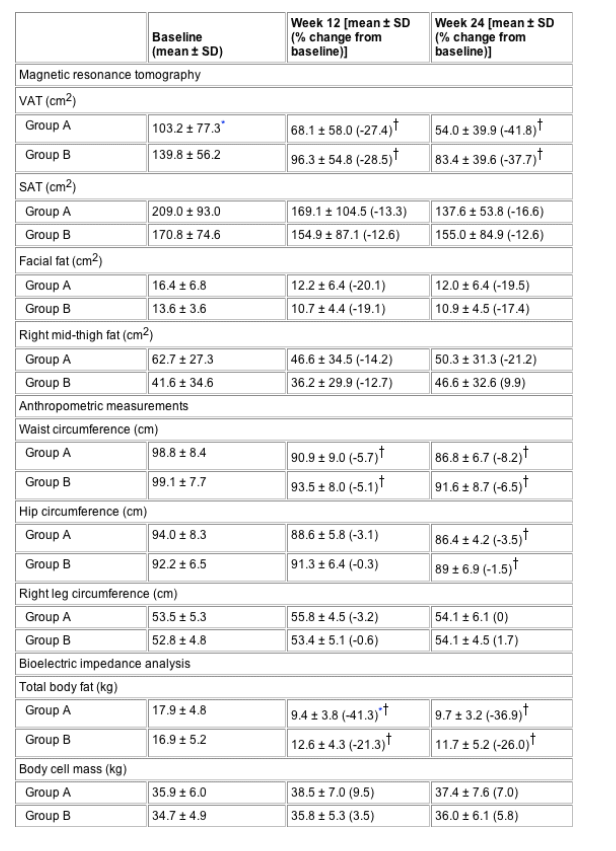
Table 3 Evolution of metabolic parameters for patients treated with 4 mg r-hGH daily (group A) compared with those treated with 4 mg r-hGH three times a week (group B) for 12 weeks (randomization phase) followed by 2 mg r-hGH daily for another 12 weeks (maintenance phase)
POSTER AT ICAAC
H-1898
Maintained Effect on Body Composition After Treatment With Recombinant
Human Growth Hormone (r-hGH) in HIV-1 Infected Patients With Lipodystrophy
46th ICAAC, San Francisco, Sept 2006
MRI sequences of the face, abdomen and at mid thigh level (MTF) were done at bsl, w12, w24 and at follow up
Median time of follow-up was 9 months (6 - 12).
At follow-up VAT remained overall 18% (+17 to -53%) below baseline
no difference between group A or B (Difference in VAT: -21 A vs B -23%).
11 pts. were treated with a PI and 5 with a NNRTI-based regimen. Response to r-hGH after 24 weeks was similar, but at follow-up the reduction of VAT remained higher in the NNRTI compared to the PI group (-26 vs -15%) (table 2).
No depletion of subcutaneous fat was seen during the follow-up period in all measured areas.
Fasting glucose, triglycerides, ASAT (sGOT) and ALAT (sGPT) were lower at follow-up compared to baseline values.
Table 2: Evolution of metabolic parameters and fat mass measured with MRI in different body regions during 24 weeks of r-hGH treatment and at follow-up 9 month after the end of treatment. Values of are shown as mean values ± standard deviation.
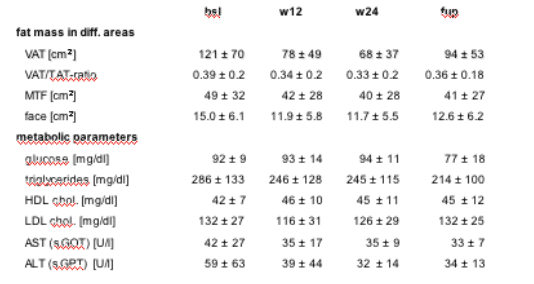
Table 3: Percentage of reduction of the visceral fat mass (VAT) compared to baseline during 24 weeks of r-hGH treatment and at follow-up 9 month after end of treatment. Values of are shown as mean values ± standard deviation

|
|
| |
| |
|
|
|