| |
HIV-Infected from Human Immunodeficiency Virus-Infected
|
| |
| |
The Journal of Clinical Endocrinology & Metabolism Dec 2006 Vol. 91, No. 12 4916-4924
Stine Johnsen, Sara E. Dolan, Kathleen V. Fitch, Jenna R. Kanter, Linda C. Hemphill, Jean M. Connelly, Robert S. Lees, Hang Lee and Steve Grinspoon
Program in Nutritional Metabolism (S.J., S.E.D., K.V.F., J.R.K., S.G.), Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114; Massachusetts General Hospital Biostatistical Center (H.L.), Boston Heart Foundation (L.C.H., J.M.C., R.S.L.), Massachusetts General Hospital, Boston, Massachusetts 02114; and Department of Infectious Diseases (S.J.), Skejby Hospital, DK-8200 Aarhus, Denmark
".....In univariate regression analysis, age, BMI, increased waist circumference, SAT, and PI use were significantly associated with carotid IMT in the HIV group. In stepwise multivariate regression analysis, age, BMI, and waist circumference were most significantly associated with IMT in the HIV-infected patients. In contrast, metabolic syndrome status per se was not associated with increased carotid IMT....
.....Increased carotid IMT thickness is a useful marker of atherosclerosis that has been shown to correlate with cardiovascular risk factors and predict stroke and myocardial infarction (10, 26, 27). A number of previous studies of carotid IMT have been performed in HIV-infected patients. A recent study of endothelial function in HIV-infected men demonstrated significant impairment of endothelial function particularly among those individuals with elevated levels of HIV replication, but no association with PI therapy was found (28). Currier et al. (12) did not show an effect of either HIV disease or PI treatment but demonstrated an effect of traditional cardiovascular parameters in regression modeling. In the Swiss cohort, Depairon et al. (11) demonstrated an increased number of plaques in PI-treated patients that could be explained by traditional risk factors. In concordance with those data, Maggi et al. (13) demonstrated increased carotid lesions in PI-treated patients. Finally, in a recent study among children, it was found that HIV infection was associated with adverse structural (IMT) and functional (endothelial reactivity) changes and that these vascular changes were more pronounced in children exposed to PI therapy (29)...."
Abstract
Context: Little is known regarding carotid intimal medial thickness (IMT) in HIV-infected women and the risk factors for subclinical atherosclerosis in this population, including antiretroviral therapy and the metabolic syndrome.
Objective: Our objective was to assess carotid IMT in relationship to HIV status and antiretroviral therapy in HIV-infected women in comparison with healthy age- and body mass index (BMI)-matched control subjects.
Setting and Subjects: The study took place at an academic medical center and included 97 HIV-infected women compared with 86 age- and BMI-matched healthy control subjects.
Main Outcome Measures: We assessed carotid IMT, metabolic syndrome, and risk factors for increased IMT.
Results:
Carotid IMT was not increased in HIV-infected women [0.62 mm (0.57-0.68); median (IQR)] compared with non-HIV-infected women [0.61 mm (0.55-0.68)] matched for age and BMI (P = 0.07) but was increased significantly among HIV patients receiving a protease inhibitor (PI) [0.65 (0.59-0.71) mm] vs. non-PI-treated patients [0.61 (0.57-0.66) mm] (P < 0.05) and vs. control subjects [0.61 (0.55-0.68) mm] (P < 0.05).
The prevalence of metabolic syndrome was significantly increased among the HIV-infected women compared with control subjects and particularly in PI- vs. non-PI-treated HIV patients (45 vs. 19%, P = 0.001). Metabolic syndrome score correlated with IMT among non-HIV patients but not among the HIV group.
Individual risk factors most strongly associated with IMT in multivariate regression modeling in the control group were age and waist-to-hip ratio, and among the HIV group age and waist circumference.
Conclusions: These data demonstrate increased carotid IMT in HIV-infected women receiving PI therapy, which may be due to associated metabolic abnormalities related to PI therapy or more direct effects of this medication class on the vasculature. Additional studies of the mechanisms by which PI uses results in subclinical atherosclerosis are needed.
Subjects and Methods
Subjects
Consecutive HIV-infected and control subjects were enrolled from March 2002 to October 2003. Exclusion criteria included use of megestrol acetate, ketoconazole, antidiabetic agents, steroids, GH, any form of estrogen (including oral contraceptive pills, oral estrogen replacement, or estrogen patch), medroxyprogesterone acetate, testosterone, or any anabolic agent within 3 months of the study or active substance abuse. These parameters were chosen to exclude use of medications known to affect body composition or specific cardiovascular risk markers, such as lipid levels or glucose. Subjects were also excluded if they were pregnant, had a BMI less than 20 kg/m2, and were less than 18 or more than 60 yr of age to avoid subjects with AIDS wasting, pediatric or elderly patients. HIV-infected subjects who had changed or initiated a new antiretroviral medication less than 2 months before the study were excluded.
Recruitment was accomplished through community advertisement and primary care provider referral. In the control subjects, HIV-negative status was verified by ELISA. Control subjects were healthy without known acute or chronic diseases. Bone density and cardiovascular risk indices as well as reproductive function have previously been reported in this cohort (14, 16, 17, 18). Testing was performed in the follicular phase in all eumenorrheic subjects. The study was approved by the Human Research Committee at the Massachusetts General Hospital. All subjects gave written informed consent at the beginning of the study.
Assessment of antiretroviral drug history and AIDS-related illnesses
Duration of HIV infection and antiretroviral medication, including all current antiretroviral medications and duration of previous antiretroviral medication use, was obtained from patient interview. Smoking history was obtained via a questionnaire.
Oral glucose challenge
Insulin and glucose were measured at 0 and 120 min after a 75-g oral glucose challenge.
Biochemical indices
Insulin levels were measured in serum using a RIA (Diagnostic Products Corp., Los Angeles, CA). Intra- and interassay coefficients of variation are 3.1-9.3 and 4.9-10.0%, respectively. Low-density lipoprotein (LDL) cholesterol was measured directly (Genzyme Diagnostics, Cambridge, MA). FSH was measured using a solid-phase immunoradiometric assay (Diagnostic Products) with an intraassay coefficient of variation of 2.2-3.8%. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, and glucose were measured using standard techniques.
Immune function
CD4+ count was determined by flow cytometry (Becton Dickinson Biosciences, San Jose, CA), and HIV viral load was determined by ultrasensitive assay (Amplicor HIV-1 Monitor Assay; Roche Molecular Systems, Branchburg, NJ) with limits of detection 50-75,000 RNA copies/ml.
Body composition
Weight and anthropometric measurements were determined in the morning, before breakfast. Abdominal visceral adipose tissue area (VAT) and sc adipose tissue areas (SAT) were assessed as previously reported, with a single-slice abdominal computed tomography (CT) scan (14).
Carotid IMT measurements
Imaging was conducted using a high-resolution 7.5-MHz phased-array transducer (Hewlett-Packard SONOS 2000/2500; Hewlett-Packard, Andover, MA). An identical procedure was previously used by our group to determine IMT in relationship to cardiovascular risk factors among an expanded group of control subjects (19). Briefly, subjects were positioned with a wedge of approximately 35 such that subject's head and torso were at an incline to reduce respiratory variation and subsequent motion in the jugulars. Imaging of the left carotid was performed with the subject turning her head 45 to the right. Imaging was performed in B-mode, and the transducer was swept in cross-section to note the position and orientation of the bifurcation of the carotid artery. The transducer was then applied to the longitudinal view, with images acquired at two angles, 90 and 45. Investigators who performed the carotid IMT were blinded as to the HIV status of the study subjects. Differences in interadventitial diameter of the carotid across the 50 frames were used to judge the cardiac cycle and select a frame of minimum diameter (diastole) as the analysis frame. Either the 90 or the 45 image in diastole was selected as the best view for image quality. The published reproducibility of the technique is excellent with a SD of 0.007 mm (20). Reproducibility was further tested and determined to be 0.004 among 10 HIV patients. The average IMT over the length of the measured segments on the left is reported.
Statistics
Continuous variables were tested for normality by use of the Wilk-Shapiro test. Carotid IMT data were not normally distributed, and thus comparisons among groups were made by the nonparametric median rank test. For noncontinuous variables, the X2 test was used. In a subanalysis comparing PI-treated and non-PI-treated patients and healthy controls, overall differences between the groups were tested by the median rank test. When significant (P < 0.05), pairwise comparisons between PI-treated and non-PI-treated and healthy control subjects were performed.
All values are reported as median with interquartile range unless otherwise indicated. Significant differences were defined as P < 0.05. The NCEP-ATPIII guidelines were used to identify patients with metabolic syndrome (21). The sum of the number of components was determined and ranged from zero (no components) to five (all the components). MS was present when at least three of five risk determinants were present. The Spearman's -correlation coefficient was determined for pairwise comparisons. All variables significantly correlated with IMT in univariate regression analysis were tested in stepwise multivariate regression analysis with a P value < 0.1 to enter the model. Final models were then constructed from the significant terms entering the models. Separate models to determine risk factors for IMT were constructed for the HIV group and healthy control subjects. Alternative models were constructed by forcing traditional cardiovascular risk parameters back into the final model. Additionally, in the HIV group, VAT was substituted for waist circumference and BMI in multivariate regression modeling. Data on use of other medications e.g. nucleoside reverse transcriptase inhibitors (NRTIs) and non-NRTIs (NNRTIs) were forced into the model. A model for the entire group was also constructed entering traditional cardiovascular risk factors. Statistical analyses were performed using JMP for SAS (version 5.1) and SAS (Cary, NC).
Results
Demographic variables
Comparison by HIV status. Ninety-seven HIV-infected subjects and 86 control subjects were included in the data analysis. The HIV-infected and control subjects recruited for this study were similar in age, BMI, and race (Table 1). Healthy controls were chosen to have a similar BMI to the HIV patients because previous studies have shown BMI to be a significant predictor of IMT in both HIV and non-HIV populations (12, 22).
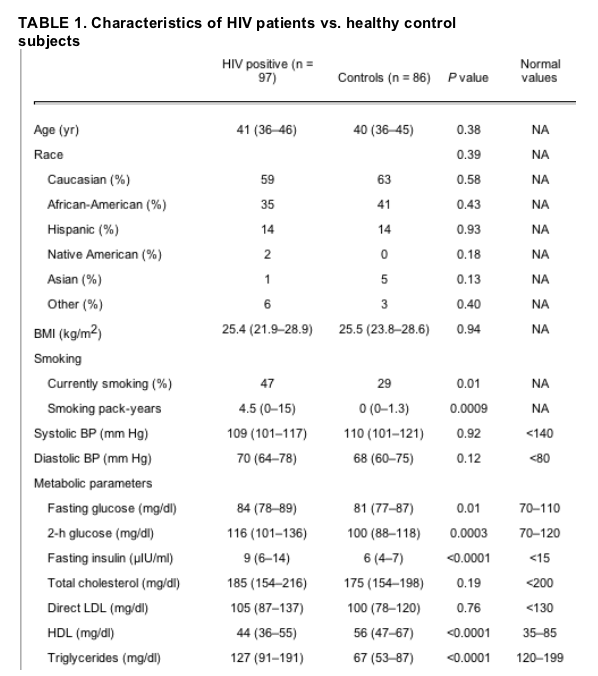
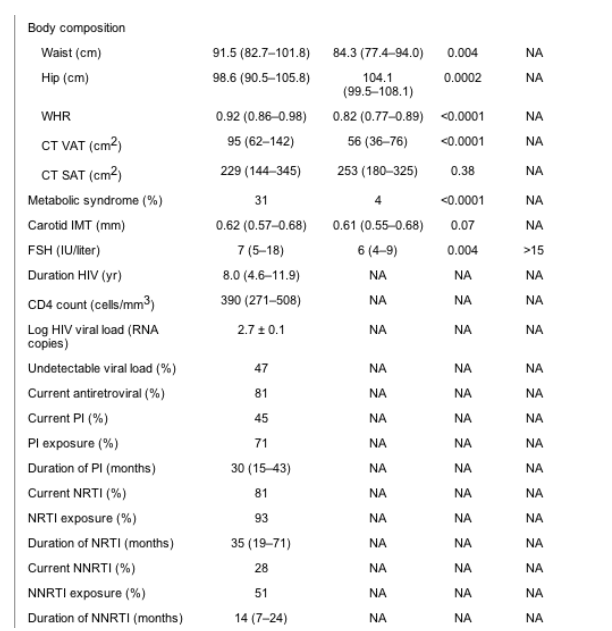
Results are [median (interquartile range)]. Specific PIs used by the study subjects were ritonavir (n = 12), lopinavir (n = 8), indinavir (n = 8), saquinavir (n = 6), and nelfinavir (n = 25). Specific NRTIs used by the study subjects were stavudine (n = 34), tenofovir (n = 2), abacavir (n = 22), lamivudine (n = 56), zidovudine (n = 31), didanosine (n = 14), and emtricitabine (n = 1). Specific NNRTIs used by the study subjects were delavirdine (n = 1), efavirenz (n = 21), and nevirapine (n = 3). For conversion from mg/dl to mmol/liter for total cholesterol, HDL-cholesterol, and LDL-cholesterol, multiply by 0.0259; to convert from mg/dl to mmol/liter for triglycerides, multiply by 0.0113; for conversion from mg/dl to mmol/liter for glucose, multiply by 0.0555; for conversion from ƒÊIU/ml to pmol/liter for insulin, multiply by 6.945. BP, Blood pressure; NA, not applicable.
A larger percentage of the HIV groups were current cigarette smokers (47 vs. 29%, P = 0.01) with higher number of pack-years smoked. FSH levels were different between HIV-infected women and control subjects (Table 1), and fewer HIV-infected women than healthy controls subjects were eumenorrheic (60 vs. 81%, P = 0.001).
Eighty-one percent of HIV-infected subjects were receiving antiretroviral medication (45% were receiving a PI, 81% were receiving an NRTI, and 28% were receiving an NNRTI). For the HIV-infected women who were not currently receiving any antiretroviral medication (n = 18), past antiretroviral exposure was as follows: 1) 33% had previous PI exposure for a mean duration of 19 (4-37) months, 2) 61% had previous NRTI exposure for a mean duration of 57 (22-81) months, and 3) 33% had previous NNRTI exposure for a mean duration of 6 (2-28) months.
Individual drugs used by the subjects are listed in the legend of Table 1. The median duration of HIV infection for HIV-positive study participants was 8.0 (4.6-11.9) yr. The median CD4 cell count was 390 (271-508) cells/mm3, and 47% of patients demonstrated undetectable viral load (Table 1).
Comparison by PI status
Forty-four HIV-infected patients were PI-treated and 53 were non-PI-treated. There was no difference in age, BMI, or race between the three groups (PI-treated, non-PI-treated, and controls). Smoking rates did not differ between PI- and non-PI-treated patients. FSH levels were similar between the two groups, and there was no difference in the percentage of subjects that were eumenorrheic (Table 2).
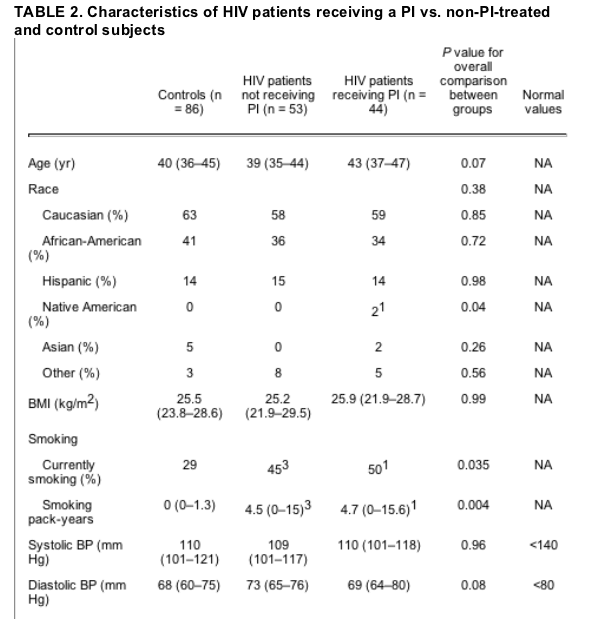
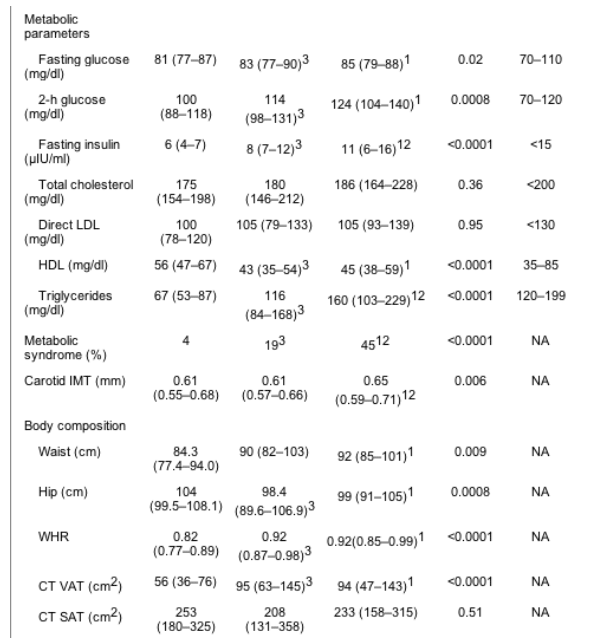
Results are [median (IQR)]. For conversion from mg/dl to mmol/liter for total cholesterol, HDL-cholesterol, and LDL-cholesterol, multiply by 0.0259; to convert from mg/dl to mmol/liter for triglycerides, multiply by 0.0113; for conversion from mg/dl to mmol/liter for glucose, multiply by 0.0555; for conversion from ƒÊIU/ml to pmol/liter for insulin, multiply by 6.945. BP, Blood pressure.
1 P < 0.05 for comparison of PI-treated patients and control subjects.
2 P < 0.05 for comparison of PI-treated patients and non-PI-treated subjects.
3 P < 0.05 for comparison of non-PI-treated patients and control subjects.
Body composition
Hip circumference was significantly lower in the HIV than the control subjects, and waist circumference as well as waist-to-hip ratio (WHR) was significantly higher in the HIV vs. control group. Abdominal visceral fat area was higher among the HIV patients compared with control subjects (Table 1).
There were no differences by PI status among HIV patients in regard to anthropometric variables (Table 2).
Biochemical indices
Fasting glucose, 2-h glucose, fasting insulin, and triglyceride concentrations were significantly higher in the HIV group compared with the control subjects. Conversely, HDL was significantly lower in the HIV group compared with the control subjects (Table 1).
Compared with non-PI-treated and controls, PI-treated patients demonstrated increased fasting insulin and increased triglyceride levels (Table 2).
Assessment of metabolic syndrome
Of the HIV-infected patients, 31% fulfilled the NCEP-ATPIII criteria for metabolic syndrome vs. 4% among the healthy controls (P < 0.0001). Sixty percent of the HIV-infected women vs. 42% of the controls (P = 0.02) demonstrated increased iliac waist, more than 88 cm; 49 vs. 6% demonstrated elevated triglycerides, more than 150 mg/dl (HIV vs. control, P < 0.0001); 69 vs. 32% demonstrated low HDL, less than 50 mg/dl (HIV vs. control, P < 0.0001); 18 vs. 8% demonstrated elevated blood pressure, more than 130/85 mm Hg or were receiving antihypertensive medication (HIV vs. control, P = 0.06); and 0 vs. 1% demonstrated elevated fasting glucose (HIV vs. control, P = 0.31). Consequently, the most prevalent features of the metabolic syndrome among the HIV patients were elevated triglycerides, low HDL, and increased waist circumference.
Comparison by PI status
The prevalence of metabolic syndrome was greater in PI-treated than non-PI-treated HIV-infected women (45 vs. 19%, P = 0.001). PI-treated women demonstrated significantly increased triglycerides compared with non-PI-treated women (63 vs. 38%, P = 0.02), and a significantly higher proportion of PI-treated women demonstrated elevated blood pressure or were receiving medication for hypertension (30 vs. 8%, P = 0.004).
Carotid IMT
Carotid IMT was not increased among the combined (PI- and non-PI-treated) HIV-infected group compared with control subjects [0.62 (0.57-0.68) vs. 0.61 (0.55-0.68) mm, P = 0.07] but was increased in PI-treated vs. non-PI-treated [0.65 (0.59-0.71) vs. 0.61 (0.57-0.66) mm, P < 0.05] and between PI-treated and healthy control subjects [0.65 (0.59-0.71) vs. 0.61 (0.55-0.68) mm, P < 0.05]. In contrast, carotid IMT was not increased in non-PI-treated women compared with healthy control subjects (Fig. 1).
FIG. 1. A and B, Results are median with interquartile range represented by the box and 10th and 90th values represented by the whiskers. In A, the white bar denotes control women and the dark gray bar denotes HIV-infected women. In B, the white bar denotes control women, the light gray bar denotes non-PI-treated women, and the dark gray bar denotes PI-treated women. In B, P = 0.006 for overall comparison between the groups by median rank test. *, P < 0.05 for comparisons between PI- and non-PI-treated and between PI-treated and control women.
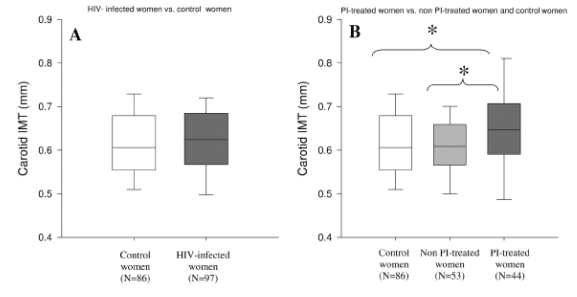
Among the HIV patients, IMT was not greater in subjects with metabolic syndrome than in those without metabolic syndrome [0.64 (0.52-0.68) vs. 0.61 (0.56-0.68) mm, metabolic syndrome vs. no metabolic syndrome, P = 0.11]. IMT was not correlated to menstrual function.
Immune function
The mean log HIV viral load was significantly higher in the non-PI-treated group. There was no difference in CD4 count between PI-treated and non-PI-treated subjects (Table 2). As expected, viral load was significantly higher in the group of non-PI-treated patients who were not receiving any medication (n = 18, 34%) compared with those receiving antiretroviral medication (n = 35, 66%) (P < 0.001).
Correlation analyses with IMT
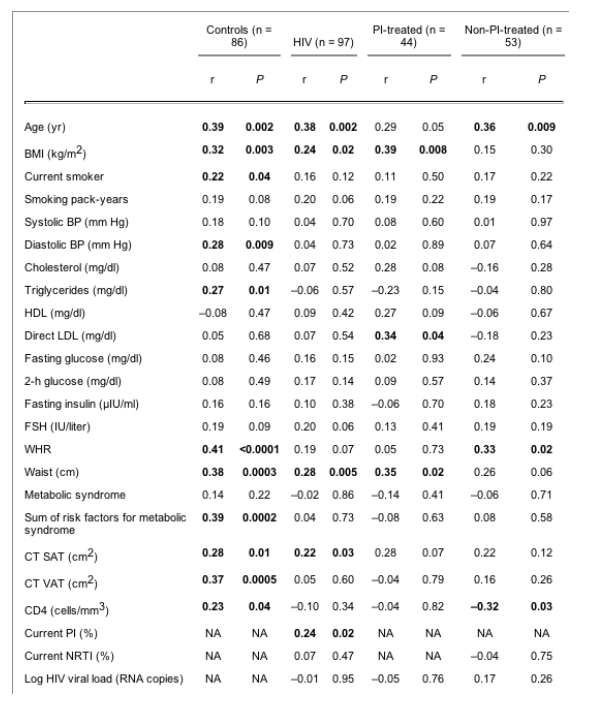
In the control subjects, IMT correlated significantly with age (r = 0.39; P = 0.002), BMI (r = 0.32; P = 0.003), smoking status (r = 0.22; P = 0.04), waist circumference (r = 0.38; P = 0.0003), WHR (r = 0.41; P < 0.0001), VAT (r = 0.37; P = 0.0005), SAT (r = 0.28; P = 0.01), diastolic blood pressure (r = 0.28; P = 0.009), and triglyceride level (r = 0.27; P = 0.01). In the controls, IMT also correlated with the number of components of the metabolic syndrome (r = 0.39; P = 0.0002). Among the HIV patients (PI- and non-PI-treated combined), IMT correlated significantly with age (r = 0.38; P = 0.002), BMI (r = 0.24; P = 0.02), current PI use (r = 0.24; P = 0.02), SAT (r = 0.22; P = 0.03), and waist circumference (r = 0.28; P = 0.005), but there was no correlation between IMT and metabolic syndrome or the number of components of the metabolic syndrome. In the group of PI-treated patients, IMT correlated with LDL (r = 0.34; P = 0.04), BMI (r = 0.39; P = 0.008), and waist circumference (r = 0.35; P = 0.02) (Table 3).
Multivariate regression analysis
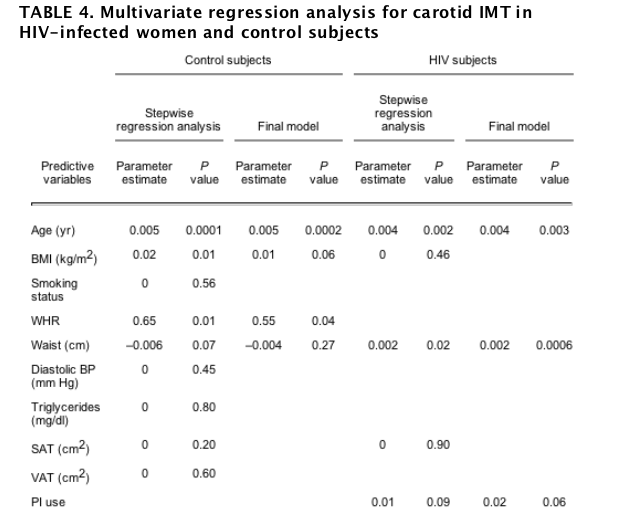
Among healthy control subjects, variables tested for initial entry into the regression model included, age, BMI, smoking status, WHR, waist circumference, SAT, VAT, diastolic blood pressure, and triglyceride level. These variables were chosen based upon significance in univariate regression analysis. Stepwise regression models are shown in Table 4. The variables in the healthy controls identified as significant by stepwise regression modeling were age, BMI, waist circumference, and WHR, but only age (β = 0.005; P = 0.002) and WHR (β = 0.55; P = 0.04) were significant in the final model, explaining 32% of the variation in IMT. Additionally, we constructed a least-squares model forcing all parameters initially identified for entry into the stepwise regression model and fasting blood glucose into the model. In this model, smoking (β = 0.0008; P = 0.93), triglyceride level (β = 0.0001; P = 0.67), diastolic blood pressure (β = 0.0006; P = 0.51), and glucose (β = -0.002; P = 0.14) were not significant, whereas age (β = 0.005; P < 0.0001), BMI (β = 0.018; P = 0.01), and WHR (β = 0.65; P = 0.03) were significant in the model, explaining 37% of the variation in IMT.
Within the HIV and control groups, stepwise regression models were constructed for factors significantly correlated to IMT in univariate regression analysis. Alternative models were constructed for the controls and HIV-infected patients, separately forcing known cardiovascular risk factors into the final models. These variables included current smoking, diastolic blood pressure (BP), triglycerides, and fasting blood sugar (see Results).
Among the HIV-infected women, variables tested for initial entry into the stepwise regression model based on univariate correlation analysis included age, BMI, waist circumference, SAT, and current PI use. The variables in the HIV group identified as significant by stepwise regression modeling were age, waist circumference, and PI use, but only age (β = 0.004; P = 0.003) and waist circumference (β = 0.002; P = 0.0006) were significant in the final model, explaining 25% of the variation in IMT (Table 4). A least-squares model was also constructed among the HIV group, forcing clinically relevant parameters such as smoking status, triglycerides, diastolic blood pressure, and fasting blood glucose into the model in addition to parameters identified by stepwise regression modeling. In this model, smoking (β = 0.01; P = 0.19), triglycerides (β = -0.0001; P = 0.07), diastolic blood pressure (β = 0.0006; P = 0.56), and fasting blood glucose (β = 0.0003; P = 0.76) were not significant, whereas age (β = 0.004; P = 0.007) and waist circumference (β = 0.002; P = 0.009) were significant, and current PI use approached statistical significance in the model (β = 0.02; P = 0.066), explaining 32% of the variation in IMT. Results were similar when variables were forced into the model one by one and as a group. Because BMI and waist circumference may relate differently to obesity in the presence of HIV lipodystrophy, we also constructed an alternative model that included only VAT, age, and current PI use. Only age and PI use entered the final model, and only age was significantly related to IMT, explaining 15% of the variation in IMT in this model. We also performed a stepwise regression analysis including NRTI and NNRTI use. Neither NRTI (β = 0; P = 0.49) nor NNRTI (β = 0; P = 0.38) use entered the final model.
A multivariate regression analysis in the combined group (HIV and non-HIV) was performed entering known cardiovascular risk factors such as age, race, body composition parameters, lipids, smoking, blood sugar and insulin, and metabolic syndrome and HIV status into stepwise regression modeling. In this model, age (β = 0.005; P < 0.0001), BMI (β = 0.006; P < 0.0001), smoking status (β = 0.02; P = 0.04), and sum of risk factors for metabolic syndrome (β = 0.02; P = 0.003) were significant.
Discussion
In this study, we demonstrate increased carotid IMT in HIV-infected women receiving a PI compared with both healthy controls and non-PI-treated women. In contrast, carotid IMT was not increased among the combined group (PI- plus non-PI-treated) of HIV-infected women compared with control subjects. The prevalence of metabolic syndrome was significantly increased among the HIV-infected women compared with healthy controls and particularly among PI-treated women. Controversy exists as to the etiology of the metabolic abnormalities and the development of the metabolic syndrome in HIV-infected patients. HIV itself, antiretroviral therapy, and associated changes in body composition may all contribute to the development of metabolic abnormalities seen in HIV-infected patients (23, 24, 25). However, our data suggest that PI use is associated with the development of common metabolic abnormalities, increasing the prevalence of the metabolic syndrome in this group.
In univariate regression analysis, age, BMI, increased waist circumference, SAT, and PI use were significantly associated with carotid IMT in the HIV group. In stepwise multivariate regression analysis, age, BMI, and waist circumference were most significantly associated with IMT in the HIV-infected patients. In contrast, metabolic syndrome status per se was not associated with increased carotid IMT.
Increased carotid IMT thickness is a useful marker of atherosclerosis that has been shown to correlate with cardiovascular risk factors and predict stroke and myocardial infarction (10, 26, 27). A number of previous studies of carotid IMT have been performed in HIV-infected patients. A recent study of endothelial function in HIV-infected men demonstrated significant impairment of endothelial function particularly among those individuals with elevated levels of HIV replication, but no association with PI therapy was found (28). Currier et al. (12) did not show an effect of either HIV disease or PI treatment but demonstrated an effect of traditional cardiovascular parameters in regression modeling. In the Swiss cohort, Depairon et al. (11) demonstrated an increased number of plaques in PI-treated patients that could be explained by traditional risk factors. In concordance with those data, Maggi et al. (13) demonstrated increased carotid lesions in PI-treated patients. Finally, in a recent study among children, it was found that HIV infection was associated with adverse structural (IMT) and functional (endothelial reactivity) changes and that these vascular changes were more pronounced in children exposed to PI therapy (29).
Carotid IMT among the female patients in our study is less than that reported in male HIV-infected patients (15, 30), suggesting that the absolute risk of coronary artery disease is lower in this group, and this likely relates to the protective effects of female gender in a largely young and premenopausal population. The clinical significance of the differences with control subject at this range of IMT is unknown. However, increased carotid IMT may further accelerate with aging, as patients with HIV live longer and become postmenopausal. Iglseder et al. (31) demonstrated that significantly more women than men fulfilled the metabolic syndrome criteria for hypertriglyceridemia and increased waist circumference, but fasting blood glucose and triglyceride levels were most strongly associated with IMT in women. In our study, there was no difference in IMT between HIV-infected women with and without the metabolic syndrome. Women in our study were younger (mean age, 41 yr) than those reported by Iglseder (mean age, 57 yr), and there may be a protective effect of premenopausal status on IMT in young women with metabolic syndrome. In contrast, the protective effects of age and gender are inadequate to mitigate the increased risk exposure from PI use and the complications that follow.
Among the HIV-infected women included in our study, low HDL, increased triglyceride, and increased waist circumference were the most prominent components of the metabolic syndrome. Similarly, Jerico et al. (32) demonstrated that hypertriglyceridemia was the most frequent trait of metabolic syndrome, followed by low HDL, high blood pressure, increased waist circumference, and high blood glucose levels in a study of HIV-infected patients, 28% of whom were women.
Previous data have demonstrated an association between metabolic syndrome and increased cardiovascular risk (33, 34). van Wijk et al. (35) demonstrated increased carotid IMT in HIV-infected men with metabolic syndrome and additionally a trend toward more pronounced endothelial dysfunction in those HIV-infected men that were on a PI-containing regimen. In our study, performed exclusively in women, traditional risk factors such as age, BMI, and waist circumference were significantly related to IMT in univariate and multivariate regression analyses among the HIV-infected patients. In contrast, hypertriglyceridemia, more prevalent among patients with HIV and particularly among those receiving a PI, was not associated with IMT in the HIV group. Furthermore, designation of metabolic syndrome status was not associated with increased IMT. It should be noted that subjects in the HIV and non-HIV groups were chosen to have a similar BMI, and thus we cannot determine the true prevalence of metabolic syndrome in a broader population of HIV patients and controls, encompassing a greater range of BMI values.
In the HIV-infected women, the percentage of current smokers and smoking pack-years were significantly higher than in the healthy controls. Smoking status, however, was associated with IMT only in the entire group and the control subjects but not among the HIV-infected women. Most previous data have shown an association between smoking status and IMT, but the association between IMT and smoking status may not be seen in studies of young patients (36).
These data demonstrate increased carotid IMT in HI V-infected women receiving PI therapy. The effects of PIs on IMT may be direct, e.g. via direct endothelial damage (37), or indirect, via effects on other risk factors, such as lipid, glucose, or body composition parameters (e.g. increased waist circumference). In this regard, PI use may be a surrogate for the numerous metabolic abnormalities, which might simultaneously contribute to increased IMT in this group. Thirty percent of the PI-treated women vs. 8% of the non-PI-treated women in our study were either on hypertensive medication or hypertensive. This corresponds well to recent reports that PI-induced lipodystrophy and metabolic abnormalities contribute to hypertension in HIV-infected patients (38, 39).
Our study demonstrates increased IMT in HIV-infected women receiving PIs compared with both healthy female controls and non-PI-treated patients. Additionally, we demonstrate increased prevalence of metabolic syndrome in HIV-infected women compared with controls but find no relationship between increased carotid IMT and metabolic syndrome in our study population of young women. The mechanisms by which PI use might increase carotid IMT in HIV-infected women are not known and may be related to traditional risk factors associated with PI use, e.g. changes in weight or body composition, or via direct effects on the vasculature. Given the increase in myocardial infarction rate seen with PI use, these questions are important and require additional study.
|
|
| |
| |
|
|
|