| |
Rosiglitazone Improved Fat Loss & Insulin in HIV+ with Insulin Resistance and Fat Redistribution
|
| |
| |
"Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio"
AIDS: Volume 21(1) 2 January 2007 p 47-57
Mulligan, Kathleena; Yang, Yangb; Wininger, David Ac; Koletar, Susan Lc; Parker, Robert Ab; Alston-Smith, Beverly Ld; Schouten, Jeffrey Te; Fielding, Roger Af; Basar, Michael Tg; Grinspoon, Stevenh
From the aUniversity of California at San Francisco, San Francisco General Hospital, San Francisco, California, USA
bStatistical and Data Analysis Center, Harvard University, Boston,
Massachusetts, USA
cOhio State University, Columbus, Ohio, USA
dNational Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA
eUniversity of Washington, Seattle, Washington, USA
fTufts University, Boston, Massachusetts, USA
gFrontier Science and Technology Research Foundation, Amherst, New York, USA
hHarvard University, Massachusetts General Hospital, Boston, Massachusetts, USA.
Notes from Jules Levin: It appears this study was only for 16 weeks yet the results find a glitazone, in this case rosiglitazone, may increase fat; this study was conducted in HIV+ individuals with fat redistribution and insulin resistance. Leg fat increased significantly on rosiglitazone. Fat increased in other areas of the body for patients on rosiglitazone but the increases were not statistically significant; again this study was only 16 weeks, too short to identify real improvements. Previously conducted studies of rosiglitazone that did not find improvements in fat may not have been conducted in a way to identify potential benefits and the patient populations that could benefit. Further studies of rosiglitazone and pioglitazone for fat loss should be conducted to identify patient populations who could benefit. A study presented at CROI 2006 and updated at the Lipodystrophy Workshop in 2006 finds pioglitazone increased fat in patients with fat loss. Pioglitazone is an anti-diabetes medication (as is rosiglitazone) and associated with the side effects of weight gain and edema. Pioglitazone appears to have a beneficial effect on lipids. The authors conclude: "thiazolidinediones may increase extremity fat in some HIV-infected patients with insulin resistance, and could be considered for use in patients with lipoatrophy and insulin resistance."
"....it is important to identify a subgroup of patients who might benefit from thiazolidinedione treatment.... Rosiglitazone negatively affected lipid profiles, as evidenced by an increase in LDL and a decrease in HDL-cholesterol.... Rosiglitazone increased concentrations of adiponectin, an adipocytokine that.....is lower in HIV-infected patients with altered fat distribution.... No treatment was associated with a significant decrease in VAT, whereas leg fat increased in subjects who received rosiglitazone compared with placebo.... thiazolidinediones may increase extremity fat in some HIV-infected patients with insulin resistance, and could be considered for use in patients with lipoatrophy and insulin resistance..... Further studies are required to determine the overall effects of thiazolidinediones and metformin on changes in fat distribution and metabolic parameters, and the implications for overall cardiovascular risk in HIV-infected patients. Such studies should identify the thiazolidinediones with the most beneficial effects on lipids and include treatment for longer than 6 months, as changes in fat distribution occur more slowly... In studies of both rosiglitazone [7,15] and pioglitazone [26], subjects who were not using thymidine analog nucleoside reverse transcriptase inhibitors had greater increases in subcutaneous fat... Accumulating data from studies in non-HIV-infected populations suggest that the negative effects of rosiglitazone on lipids may be unique to this thiazolidinedione, and contrast with the positive effects with pioglitazone [31,32]. In a preliminary report of a placebo-controlled study in patients with HIV infection [26], HDL-cholesterol increased in subjects receiving pioglitazone....
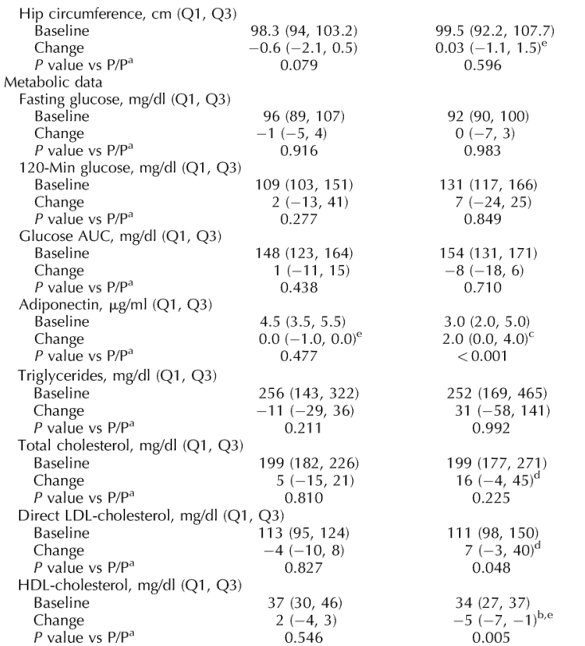
Note from Jules Levin: in this study, Leg fat increased in the rosiglitazone group and the change was statistically significant compared to placebo. If you look at the table below fat decreased in various body areas in the metformin group (not statistically significant) but fat increased for patients receiving rosiglitazone (not statistically significant). Increases were 3.3% in subcutaneous abdominal fat (CT), 0.2% in total body fat (DEXA), total extremity fat (DEXA) by 4%, arm fat by 3.7%, leg fat (DEXA) by 4.8% (p=0.034). Triglycerides and total cholesterol also increased (not statistically significant), LDL increased (p=0.048) and HDL decreased (p=0.005) on rosiglitazone (see table below).
"....This study was undertaken to evaluate the effects of two different classes of insulin-sensitizing agents on fat distribution and insulin sensitivity in HIV-infected patients with objective evidence of alterations in both fat distribution and glucose metabolism. Insulin resistance and hyperinsulinemia are known to be independent risk factors for cardiovascular disease in non-HIV-infected patients [22], and therapies that improve insulin sensitivity may reduce the cardiovascular risk in patients with HIV infection...
.....There has been less consistency among published studies on the effects of rosiglitazone on subcutaneous fat in HIV-infected individuals with lipoatrophy. As a class, thiazolidinediones stimulate adipogenesis via effects on PPARγ [23]. Troglitazone increased subcutaneous fat in clinical studies in non-HIV-infected individuals [8-11]. In patients with HIV infection, two randomized studies found no significant differences in the changes in subcutaneous abdominal fat with rosiglitazone, compared with placebo [14,15]. In contrast, Hadigan et al. [13] reported that rosiglitazone increased subcutaneous leg fat (P = 0.02 versus placebo), consistent with the observed increase in leg fat in the current study (P = 0.032 versus placebo). Increases in subcutaneous abdominal fat were also reported by Van Wijk et al. [7] (P < 0.05 versus baseline and P = 0.007 versus metformin); and Gelato et al. [12] (P = 0.05 versus baseline). Taken together, these results suggest that thiazolidinediones have the potential to increase subcutaneous fat in some HIV-infected subjects. There have been preliminary attempts to identify factors associated with a positive response. For example, van Wijk et al. [7] reported a significant correlation between baseline insulin AUC and the change in SAT and modest negative correlations between baseline weight and fat and increases in SAT during treatment. Another potential factor may be the use of thymidine analog nucleoside reverse transcriptase inhibitors, which have been reported to downregulate PPARγ expression in the adipose tissue of healthy volunteers [24] and during rosiglitazone treatment in HIV-infected individuals with lipoatrophy [25]. In studies of both rosiglitazone [7,15] and pioglitazone [26], subjects who were not using thymidine analog nucleoside reverse transcriptase inhibitors had greater increases in subcutaneous fat. To date, the only pharmacological strategy that consistently reverses lipoatrophy involves switching from a thymidine-containing to a thymidine-sparing antiretroviral regimen [27-30]. However, even partial reversal of lipoatrophy with such switches occurs slowly. Therefore, whereas the search for other viable therapies for lipoatrophy continues, it is important to identify a subgroup of patients who might benefit from thiazolidinedione treatment.....
....Rosiglitazone may increase subcutaneous fat in some individuals....No treatment was associated with significant changes in visceral or subcutaneous abdominal fat. Leg fat increased in subjects on Rosi/P compared with placebo....Total fat tended to decrease in Met/P (P = 0.070 versus baseline). There were no significant changes in trunk, total extremity, or arm fat in any treatment group. However, leg fat increased in the Rosi/P group, and this change was statistically significant when compared with placebo
... During treatment, neither VAT nor SAT changed significantly in any group, and no differences between treatment groups and placebo were statistically significant. Weight decreased significantly in both groups randomly assigned to metformin....in Met/P, the decrease was statistically significant compared with placebo...
....the insulin AUC decreased significantly compared with baseline in both groups randomly assigned to receive rosiglitazone [Rosi/P -25.7 (-48.5, 20.5) μIU/ml, P = 0.012; Met/Rosi -17.7 (-68.9, -3.5) μIU/ml, P = 0.002; Fig. 2a]; and tended to decrease in the Met/P group [-11.1 (-31.2, 5.9) μIU/ml, P = 0.058]...."
Abstract
Objective: To evaluate the effects of metformin and rosiglitazone, alone or in combination, on fat distribution, insulin sensitivity, and lipids in HIV-infected patients with insulin resistance and changes in fat distribution.
Methods: A total of 105 subjects were randomly assigned to receive metformin (500 mg twice a day increasing to 1000 mg twice a day after 2 weeks) with rosiglitazone placebo (Met/P, N = 26); rosiglitazone (4 mg/day) with metformin placebo (Rosi/P, N = 27); rosiglitazone (4 mg/day) plus metformin (500 mg twice a day increasing to 1000 mg twice a day after 2 weeks; Met/Rosi, N = 25); or dual placebo (P/P, N = 27) for 16 weeks. Efficacy assessments included oral glucose tolerance testing, abdominal computed tomography, whole-body dual-energy X-ray absorptiometry, and the measurement of fasting lipids and other biochemical indices. Safety was monitored throughout. Intent-to-treat analyses were performed using non-parametric methods.
Results: The median insulin area under the curve (AUC) decreased significantly compared with baseline in both groups randomly assigned to rosiglitazone (Rosi/P -25.7 μIU/ml, P = 0.012; Met/Rosi -17.7 μIU/ml, P = 0.002); and tended to decrease in the Met/P group (-11.1 μIU/ml, P = 0.058). The change in AUC with combination therapy was significant compared with placebo (P = 0.032). No treatment was associated with significant changes in visceral or subcutaneous abdominal fat. Leg fat increased in subjects on Rosi/P compared with placebo (+4.8 versus -8.3%, P = 0.034). Rosiglitazone, but not metformin, increased adiponectin but also increased LDL-cholesterol and decreased HDL-cholesterol. Gastrointestinal effects occurred frequently in subjects on metformin.
Conclusion: Both treatments improved insulin sensitivity, but neither reduced visceral fat. Rosiglitazone may increase subcutaneous fat in some individuals.
Introduction
Metabolic and body composition changes, including insulin resistance, dyslipidemia, and a loss of subcutaneous fat, are common among HIV-infected patients receiving HAART [1]. Some patients with HIV infection also have increased abdominal visceral fat. These metabolic and morphological abnormalities may contribute to an increased risk of cardiovascular disease. Insulin-sensitizing agents may be useful treatments for insulin resistance and abnormal fat distribution among HIV-infected patients. Insulin-sensitizing agents can delay the development of diabetes in non-HIV-infected patients with insulin resistance and impaired glucose tolerance [2], and may improve cardiovascular risk. Furthermore, such agents may improve the abnormal fat distribution and altered inflammatory adipocytokine levels in patients with HIV infection.
Earlier studies have suggested that the biguanide metformin promotes weight loss while improving insulin sensitivity in seronegative individuals [3,4], and may reduce visceral fat and insulin resistance among HIV-infected patients [5-7]. Another class of insulin-sensitizing agents, the thiazolidinediones, has also been studied. Troglitazone, a thiazolidinedione that is no longer available, decreased visceral fat while increasing subcutaneous fat in patients with non-HIV lipodystrophy [8], type 2 diabetes mellitus [9,10], or visceral obesity [11]. Some studies in HIV-infected patients have suggested that currently available thiazolidinediones, including rosiglitazone, may increase subcutaneous fat and improve insulin sensitivity and other important biochemical markers, including adiponectin [7,12,13]. However, other studies showed no difference between rosiglitazone and placebo in changes in subcutaneous fat [14,15].
Metformin and the thiazolidinediones improve insulin sensitivity by different mechanisms, with metformin working primarily by improving hepatic insulin sensitivity and the thiazolidinediones by enhancing peripheral insulin-stimulated glucose uptake through the activation of peroxisome proliferator-activator receptor gamma (PPARγ) [16]. These agents have additive effects on glycemic control when used together in seronegative populations [17,18]. Although studies have compared metformin and a thiazolidinedione in patients with HIV infection [7,19], to our knowledge no study has examined the combination or included a placebo control group. The present study was designed to evaluate the effects of metformin and rosiglitazone, alone or in combination, in HIV-infected patients with insulin resistance and changes in fat distribution. The primary endpoints were a change in fasting insulin, insulin area under the curve (AUC), visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) areas by computed tomography (CT), and safety. Secondary endpoints included regional fat by dual-energy X-ray absorptiometry (DEXA), fasting glucose and glucose AUC, adiponectin, and lipid levels.
Methods
Subjects
A total of 105 subjects with documented HIV-1 infection were recruited from 28 AIDS Clinical Trials Group (ACTG) sites across the United States (Fig. 1). Individuals were eligible if they had evidence of insulin resistance, as defined by either a fasting serum insulin level of 15 μIU/ml or greater or 2-h insulin of 75 μIU/ml or greater after a 75-g glucose load, or 2-h glucose greater than 140 mg/dl after a 75-g glucose load and fasting serum insulin level of 10 μIU/ml or greater; and self-reported changes in fat distribution with a waist/hip ratio more than 0.95 for men or more than 0.85 for women, or a waist circumference greater than 100 cm. We chose a criterion for fasting insulin based on data from the Framingham Study (J. Meigs, personal communication), so the cutoff of 15 μIU/ml represents the 90th percentile of a large normative database. All subjects were on a stable antiretroviral regimen for 60 days or more, with an HIV-1-RNA level of less than 10 000 copies/ml. Women on estrogen preparations or men on testosterone replacement were eligible if dosing was stable for 6 months before enrollment. Exclusion criteria included hemoglobin less than 9.1 and 8.9 g/dl for men and women, respectively; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than 2.5 X the upper limit of normal (ULN); total bilirubin greater than 2.5 X ULN; creatinine greater than 1.4 mg/dl; lactate greater than 1.5 X ULN; fasting glucose greater than 126 mg/dl; total testosterone levels in men below normal; pregnant women; body mass index less than 18 or over 40 kg/m2; or the use of antidiabetic medications, growth hormone, supraphysiological glucocorticoids or testosterone, other anabolic steroids, appetite stimulants, and immune modulators. The study was approved by each site's institutional review board, and all subjects gave written informed consent.
Study design
Eligible individuals were randomly assigned to receive metformin (500 mg twice a day increasing to 1000 mg twice a day after 2 weeks) with rosiglitazone placebo (Met/P, N = 26); rosiglitazone (4 mg/day) with metformin placebo (Rosi/P, N = 27); rosiglitazone (4 mg/day) plus metformin (500 mg twice a day increasing to 1000 mg twice a day after 2 weeks; Met/Rosi, N = 25); or dual placebo (P/P, N = 27) for 16 weeks. All medications were taken by mouth. Randomization was stratified for sex.
Metformin and the matching placebo were supplied by Bristol-Myers Squibb Company (Plainsboro, New Jersey, USA). Rosiglitazone was initially purchased by the ACTG, and blinded rosiglitazone and matching placebo capsules were formulated by the NIAID Clinical Research Products Management Center. Subsequently, blinded rosiglitazone and placebo were provided by GlaxoSmithKline (Research Triangle Park, North Carolina, USA).
Procedures
At baseline, fasting blood samples were collected for hematology, chemistries, liver function tests (LFT), lactate, CD4 cell count, and pregnancy testing in women of childbearing potential; plasma and serum were stored for batch analyses of lipids, adiponectin, and HIV-1 RNA. An oral glucose tolerance test was performed with blood samples drawn for glucose and insulin before and 30, 60, 90, and 120 min after the consumption of a 75 g glucose solution, with AUC calculated by the trapezoid method divided by the time in minutes (μIU/ml min). Subjects had a physical examination and updated medical history. Transverse single-slice CT scans of the abdomen (L4-L5) and whole-body DEXA scans were obtained using standardized protocols and were analysed centrally at Tufts University by a technician who was blinded to treatment assignment. For DEXA scans, a standard phantom was scanned at each site with each scanner used in the study. The same scanners at each site were used for all evaluations on any individual subject when possible. Weight and waist and hip circumferences were measured following standardized ACTG procedures. Subjects reviewed 72-h food intake diaries with a dietician and completed a bidirectional body image questionnaire.
During the study, subjects underwent safety evaluations at weeks 2, 4, 8, 12, and 16 that included measurements of lactate, LFT, hematology, chemistries, pregnancy testing when indicated, and a targeted physical examination and updated history. At week 8, fasting samples were collected and stored and the oral glucose tolerance test was repeated. The week 16 visit was identical to baseline. Subjects who discontinued study medication as a result of toxicity continued to be followed (off-study drug, on study).
Safety monitoring
Dose reductions were allowed for metformin/metformin placebo (1000 to 500 mg twice a day) but not for rosiglitazone/rosiglitazone placebo. Because of the potential risk of lactic acidosis with metformin and liver toxicity with thiazolidinediones [20], the protocol included strict toxicity management guidelines. All subjects were counselled regarding the signs and symptoms of lactic acidosis. Study medications were held for lactate levels of 1.5-2.0 X ULN, LFT greater than 2.5 ULN, and grade 2 diarrhea, nausea, or vomiting; and permanently discontinued for lactate greater than 2.0 X ULN, LFT greater than 3.0 X ULN, creatinine greater than 2.0 mg/dl, grade 3 diarrhea or greater, nausea, or vomiting, blood glucose less than 40 or more than 200 mg/dl, or grade 2 anemia or greater. Biannual safety reports were prepared for review by a data and safety monitoring board.
Laboratory methods
Batched samples were stored centrally at -70 and analysed at the end of the study. Glucose concentrations were measured using the hexokinase technique in plasma collected on sodium fluoride/potassium oxalate. Serum insulin was measured using an immunometric assay with no cross-reactivity with pro-insulin (DPC Immulite 2000, Diagnostic Products Corp., Los Angeles, California, USA). Triglycerides and total and HDL-cholesterol levels, as well as direct LDL-cholesterol, were measured using enzymatic techniques. All of the foregoing measurements were performed at Quest Diagnostics (Baltimore, Maryland, USA). Adiponectin was measured by enzyme-linked immunoassay and free fatty acids by an in-vitro colorimetric method at Nichols Institute (San Juan Capistrano, California, USA). HIV-1 RNA was measured at Johns Hopkins University using the Roche Amplicor HIV-1 Ultrasensitive Assay (Roche, Indianapolis, Indiana, USA).
Statistics: sample size and accrual
The study was powered to observe a difference between any active arm and the P/P arm of six units in the change of fasting insulin after 16 weeks of treatment. Using available data [21], the coefficient of variation of the change in fasting insulin for a wide range of effect sizes was assumed to be unity. Using a standard t-test approximation along with a Pitman efficiency of 0.864, it was estimated that 25 evaluable subjects per group would be required to detect this difference with 80% power and an overall alpha of 0.05, adjusted for the three comparisons (Met/P versus P/P, Rosi/P versus P/P, and Met/Rosi versus P/P) that constitute the primary hypothesis. A 20% loss-to-follow-up rate was planned, with a final target of 32 subjects in each arm. The first subject was enrolled in September, 2001. In February, 2004, the data and safety monitoring board reviewed data from the first 98 subjects. They concluded that recruitment to the originally specified target would not significantly improve the ability of the study to achieve its primary endpoints, and recommended that the study close to accrual. They noted that there were no safety concerns and that the study retained significant scientific merit; thus subjects already enrolled were allowed to complete the study. The final subject was enrolled in March, 2004.
Statistical analysis
The data were analysed primarily by intent-to-treat. One subject in the Rosi/P group never started study medication and was excluded from all but the baseline analyses. Non-parametric methods were used for statistical tests. Changes within each treatment group were evaluated using the Wilcoxon signed rank test. For comparisons between treatment groups, the Kruskal-Wallis test was used for continuous outcomes and Fisher's exact test for categorical outcomes. Reported results did not control for sex, but Van Elteren's test controlling for sex showed similar results for primary endpoints. Intent-to-treat results were confirmed in as-treated analyses. All statistical tests were two-sided with the significance level at 0.05. No adjustment was made for multiple tests. All the analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina, USA). The data reported are medians with interquartile ranges (Q1, Q3), unless indicated otherwise.
Results
Subjects
Of the 105 subjects enrolled, two-thirds were men and approximately two-thirds were Caucasian (Table 1). At baseline, there were no significant differences between the groups in demographic, disease-related, or metabolic indices. Although the study did not specifically recruit individuals with lipoatrophy, approximately 50% of subjects reported loss of tissue in the legs at enrollment (data not shown).
Insulin sensitivity
At baseline, there were no significant differences between the groups in insulin AUC or fasting or 120-min insulin levels (Table 1). During treatment, the insulin AUC decreased significantly compared with baseline in both groups randomly assigned to receive rosiglitazone [Rosi/P -25.7 (-48.5, 20.5) μIU/ml, P = 0.012; Met/Rosi -17.7 (-68.9, -3.5) μIU/ml, P = 0.002; Fig. 2a]; and tended to decrease in the Met/P group [-11.1 (-31.2, 5.9) μIU/ml, P = 0.058]. The change in AUC in the group that received combination therapy was statistically significant when compared with placebo (P = 0.032). Changes in insulin AUC in the single-treatment groups did not achieve a level of statistical significance compared with placebo (P = 0.141 and P = 0.073 for Met/P and Rosi/P, respectively). Following a similar pattern, changes in fasting insulin tended to decrease in all three treatment groups [Met/P -2 (-5, 1) μIU/ml, P = 0.068; Rosi/P -4 (-11, 2) μIU/ml, P = 0.082; Met/Rosi -4 (-9, 1) μIU/ml, P = 0.105]. However, none achieved a level of statistical significance when compared with either baseline or placebo (Fig. 2b).
Abdominal fat, weight, and body composition
At baseline, there were no significant differences between the groups in weight, VAT or SAT by CT, or total or regional fat by DEXA (Table 2). During treatment, neither VAT nor SAT changed significantly in any group, and no differences between treatment groups and placebo were statistically significant. Weight decreased significantly in both groups randomly assigned to metformin (P < 0.001 and P = 0.002 versus baseline in Met/P and Met/Rosi, respectively); in Met/P, the decrease was statistically significant compared with placebo (P = 0.029), and in Met/Rosi the decrease approached but did not achieve a level of statistical significance compared with placebo (P = 0.056). Total fat tended to decrease in Met/P (P = 0.070 versus baseline). There were no significant changes in trunk, total extremity, or arm fat in any treatment group. However, leg fat increased in the Rosi/P group, and this change was statistically significant when compared with placebo (P = 0.034; 95% confidence interval of difference versus placebo 0.8-17.3%). Hip circumference decreased significantly in Met/Rosi (P = 0.001 and P = 0.002 versus baseline and placebo, respectively). The waist/hip ratio did not change in any group (data not shown).
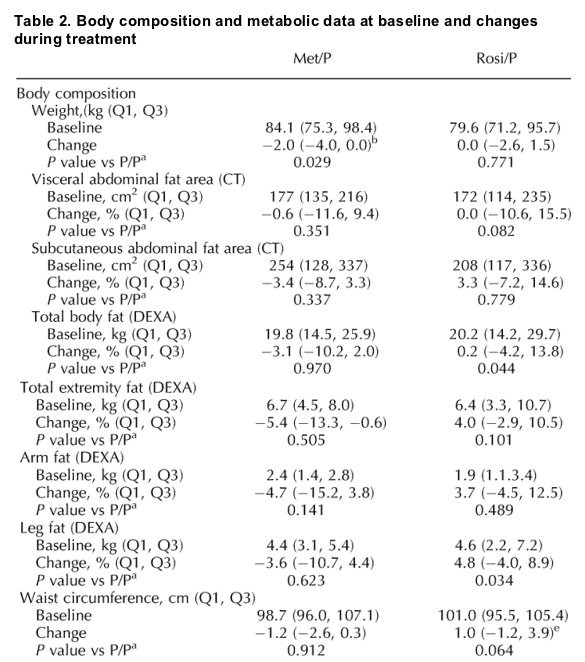
Glucose, lipids, and other metabolic measurements
Fasting and 120-min glucose levels and glucose AUC neither differed at baseline nor changed significantly during treatment (Table 2). Adiponectin levels increased significantly in both groups on rosiglitazone, compared with both baseline and placebo, but not in the Met/P group (Table 2). The difference between Met/Rosi and Met/P in the change in adiponectin was also highly significant (P < 0.001).
There were no significant differences between the groups in lipids at baseline (Table 2). During treatment, there were no significant differences in changes in triglycerides, total cholesterol, or free fatty acids in any treatment group, compared with P/P. However, in the Rosi/P group, LDL-cholesterol increased (P = 0.030 and P = 0.048 versus baseline and P/P, respectively), and HDL-cholesterol decreased (P < 0.001 and P = 0.005, respectively). These effects appear to have been nullified by the co-administration of metformin with rosiglitazone.
Self-reported data
Energy intake was not different at baseline (data not shown). During the study, the overall energy intake tended to decrease, but there were no significant differences in the change in total energy or macronutrient intake in any treatment group compared with placebo (data not shown). Likewise, there were no significant differences in the change in the self-rating of body shape during treatment (data not shown).
Safety
Safety and toxicity were evaluated using both clinical symptoms and laboratory measures. Gastrointestinal symptoms were most common (Table 3). Diarrhea was the most frequently reported adverse event, and occurred in 65 and 52% of subjects in the Met/P and Met/Rosi groups, both significantly more often than in those on placebo (P = 0.001 and P = 0.007, respectively). Most cases were classified as mild or moderate, with only one case of severe diarrhea in the dual-therapy arm. Combination therapy also caused more diarrhea than rosiglitazone alone (P = 0.001) and more vomiting than placebo (P = 0.023).
There were no significant differences between the groups in individual safety laboratory studies, including hemoglobin, fasting glucose, serum creatinine, LFT or lactate. The majority of the increases in lactate were relatively mild (1.5-2.0 X ULN). CD4 cell counts and HIV-1-RNA levels remained stable.
Dose modifications occurred in 46 out of 105 subjects (44%), with the majority ultimately resulting in the permanent discontinuation of study medication. There was a significant difference between the groups in the proportion of subjects who permanently discontinued treatment prematurely (P = 0.02), with the greatest number occurring in the Met/P group (Table 3).
Discussion
This study was undertaken to evaluate the effects of two different classes of insulin-sensitizing agents on fat distribution and insulin sensitivity in HIV-infected patients with objective evidence of alterations in both fat distribution and glucose metabolism. Insulin resistance and hyperinsulinemia are known to be independent risk factors for cardiovascular disease in non-HIV-infected patients [22], and therapies that improve insulin sensitivity may reduce the cardiovascular risk in patients with HIV infection. As expected, there was evidence of improved insulin sensitivity with each treatment and the combination, as seen in reductions in the insulin AUC, and a tendency for fasting insulin levels to decrease. These results are consistent with those of other randomized studies in patients with HIV infection [5-7,13-15]. The reduction in insulin AUC was statistically significant compared with placebo only for the group who received combination therapy, suggesting that there may have been a more consistent effect in this group. However, the median changes in insulin AUC or fasting insulin achieved with combination therapy were not statistically significant compared with single-agent therapy. The premature termination of this study may have limited our power to determine whether combination therapy had an additive effect on insulin sensitivity, compared with single-agent therapy.
Consistent with results in studies in HIV-infected [5-7] and uninfected [3,4] populations, treatment with metformin was associated with significant decreases in weight. Although total body fat also decreased in the Met/P group when compared with placebo, there was no concurrent reduction in VAT. This result was unexpected. In randomized open-label studies in HIV-infected subjects using the maximum daily dose of metformin (total 2.5 g/day), significant reductions in VAT were reported when compared with an observation-only group [5], or subjects who received rosiglitazone and experienced no change in VAT [7]. In a randomized, placebo-controlled study using a lower dose of metformin (total 1 g/day), Hadigan et al. [6] reported a trend towards decreased VAT (P = 0.08 versus placebo). In the present study, we used an intermediate dose of metformin (total 2 g/day) but saw no reduction in VAT. This result may be partly related to high rates of temporary and permanent discontinuation and dose reductions in the Met/P group, such that overall exposure to metformin was less than intended, even among subjects who were on treatment at the end of the study period.
Rosiglitazone did not reduce VAT. Troglitazone, an earlier generation thiazolidinedione subsequently removed from the market by the US Food and Drug Administration, decreased VAT in non-HIV-infected patients [8]. The current study was designed to determine whether the positive effects of troglitazone on VAT could also be achieved with rosiglitazone. Although one small, open-label study reported that rosiglitazone decreased VAT in HIV-infected patients [12], larger, randomized studies have reported no significant effects on VAT [7,13-15]. It remains unclear whether the reductions in VAT achieved with troglitazone were specific to that agent or whether there are factors related to HIV or its therapies that prevent thiazolidinediones from reducing VAT in this population.
There has been less consistency among published studies on the effects of rosiglitazone on subcutaneous fat in HIV-infected individuals with lipoatrophy. As a class, thiazolidinediones stimulate adipogenesis via effects on PPARγ [23]. Troglitazone increased subcutaneous fat in clinical studies in non-HIV-infected individuals [8-11]. In patients with HIV infection, two randomized studies found no significant differences in the changes in subcutaneous abdominal fat with rosiglitazone, compared with placebo [14,15]. In contrast, Hadigan et al. [13] reported that rosiglitazone increased subcutaneous leg fat (P = 0.02 versus placebo), consistent with the observed increase in leg fat in the current study (P = 0.032 versus placebo). Increases in subcutaneous abdominal fat were also reported by Van Wijk et al. [7] (P < 0.05 versus baseline and P = 0.007 versus metformin); and Gelato et al. [12] (P = 0.05 versus baseline). Taken together, these results suggest that thiazolidinediones have the potential to increase subcutaneous fat in some HIV-infected subjects. There have been preliminary attempts to identify factors associated with a positive response. For example, van Wijk et al. [7] reported a significant correlation between baseline insulin AUC and the change in SAT and modest negative correlations between baseline weight and fat and increases in SAT during treatment. Another potential factor may be the use of thymidine analog nucleoside reverse transcriptase inhibitors, which have been reported to downregulate PPARγ expression in the adipose tissue of healthy volunteers [24] and during rosiglitazone treatment in HIV-infected individuals with lipoatrophy [25]. In studies of both rosiglitazone [7,15] and pioglitazone [26], subjects who were not using thymidine analog nucleoside reverse transcriptase inhibitors had greater increases in subcutaneous fat. To date, the only pharmacological strategy that consistently reverses lipoatrophy involves switching from a thymidine-containing to a thymidine-sparing antiretroviral regimen [27-30]. However, even partial reversal of lipoatrophy with such switches occurs slowly. Therefore, whereas the search for other viable therapies for lipoatrophy continues, it is important to identify a subgroup of patients who might benefit from thiazolidinedione treatment.
Rosiglitazone negatively affected lipid profiles, as evidenced by an increase in LDL and a decrease in HDL-cholesterol. Interestingly, the co-administration of metformin nullified these deleterious effects. Accumulating data from studies in non-HIV-infected populations suggest that the negative effects of rosiglitazone on lipids may be unique to this thiazolidinedione, and contrast with the positive effects with pioglitazone [31,32]. In a preliminary report of a placebo-controlled study in patients with HIV infection [26], HDL-cholesterol increased in subjects receiving pioglitazone.
Rosiglitazone increased concentrations of adiponectin, an adipocytokine that is associated inversely with VAT and positively with insulin sensitivity [33], and is lower in HIV-infected patients with altered fat distribution [34,35]. Adiponectin increases fatty acid oxidation, thereby improving glucose transport into muscle [36]. The increase in adiponectin may partly explain the improvement in insulin resistance with rosiglitazone observed in this study. Low adiponectin concentrations have been shown to predict cardiovascular disease in non-HIV-infected adults [37]. Therefore, an improvement in adiponectin may be an independent benefit of rosiglitazone, but must be evaluated in the context of the adverse effects on lipids.
In summary, the present study was the first placebo-controlled comparison of the effects of metformin and a thiazolidinedione, individually and in combination, in HIV-infected subjects. As expected, all three groups who received active treatment experienced improvements in insulin sensitivity, which may be beneficial in terms of cardiovascular risk in such patients. No treatment was associated with a significant decrease in VAT, whereas leg fat increased in subjects who received rosiglitazone compared with placebo. Rosiglitazone is not an effective treatment for excess VAT in HIV-infected patients. Rosiglitazone, but not metformin, had adverse effects on lipids, but improved adiponectin. The current study informs clinicians as to the positive effects of insulin-sensitizing agents on indices of insulin resistance, even among HIV-infected patients receiving concomitant therapy with insulin-antagonizing agents such as protease inhibitors. Metformin promotes weight loss, and may be useful for overweight insulin-resistant patients with HIV infection. In contrast, thiazolidinediones may increase extremity fat in some HIV-infected patients with insulin resistance, and could be considered for use in patients with lipoatrophy and insulin resistance.
Further studies are required to determine the overall effects of thiazolidinediones and metformin on changes in fat distribution and metabolic parameters, and the implications for overall cardiovascular risk in HIV-infected patients. Such studies should identify the thiazolidinediones with the most beneficial effects on lipids and include treatment for longer than 6 months, as changes in fat distribution occur more slowly. Furthermore, future studies may utilize lower, but potentially equally effective, doses of metformin that are better tolerated than the dose chosen for the present study. Importantly, future studies should determine whether the use of these agents, alone and in combination, can reduce the risk of diabetes and cardiovascular disease in patients with HIV infection.
References
1. Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005; 352:48-62.
[Medline Link] [CrossRef] [Context Link]
2. Knowler WC, Barrett-Connor E, Fowler WE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393-403.
[Context Link]
3. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med 1995; 333:550-554.
[Medline Link] [CrossRef] [Context Link]
4. Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res 1998; 6:47-53.
[Medline Link] [Context Link]
5. Saint-Marc T, Touraine JL. Effects of metformin on insulin resistance and central adiposity in patients receiving effective protease inhibitor therapy. AIDS 1999; 13:1000-1002.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
6. Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: a randomized controlled trial. JAMA 2000; 284:472-477.
[Medline Link] [CrossRef] [Context Link]
7. van Wijk JP, de Koning EJ, Cabezas MC, Roodt RJ, Joven J, Rabelink TJ, et al. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: a randomized trial. Ann Intern Med 2005; 143:337-346.
[Medline Link] [Context Link]
8. Arioglu E, Duncan-Morin J, Sebring N, Rother KI, Gottlieb N, Lieberman J, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med 2000; 133:263-274.
[Medline Link] [Context Link]
9. Mori Y, Murakawa Y, Okada K, Horikoshi H, Yokoyama J, Tajia N, et al. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care 1999; 22:908-912.
[Medline Link] [Context Link]
10. Kawai T, Takei I, Oguma Y, Ohashi N, Tokui M, Oguchi S, et al. Effects of troglitazone on fat distribution in the treatment of male type 2 diabetes. Metabolism 1999; 48:1102-1107.
[Medline Link] [CrossRef] [Context Link]
11. Nakamura T, Funahashi T, Yamashita S, Nishida M, Nishida Y, Takahashi M, et al. Thiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation - double-blind placebo-controlled trial. Diabetes Res Clin Pract 2001; 54:181-190.
[Medline Link] [CrossRef] [Context Link]
12. Gelato MC, Mynarcik DC, Quick JL, Steigbigel RT, Fuhrer J, Brathwaite CE, et al. Improved insulin sensitivity and body fat distribution in HIV-infected patients treated with rosiglitazone: a pilot study. J Acquir Immune Defic Syndr 2002; 31:163-170.
[Fulltext Link] [Medline Link] [Context Link]
13. Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized, controlled trial. Ann Intern Med 2004; 140:786-794.
[Medline Link] [Context Link]
14. Sutinen J, Hakkinen AM, Westerbacka J, Seppala-Lindroos A, Vehkavaara S, Halavaara J, et al. Rosiglitazone in the treatment of HAART-associated lipodystrophy - a randomized double-blind placebo-controlled study. Antivir Ther 2003; 8:199-207.
[Context Link]
15. Carr A, Workman C, Carey D, Rogers G, Martin A, Baker D, et al. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo-controlled trial. Lancet 2004; 363:429-438.
[Medline Link] [CrossRef] [Context Link]
16. Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 2004; 53:2169-2176.
[Medline Link] [Context Link]
17. Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med 1998; 338:867-872.
[Medline Link] [CrossRef] [Context Link]
18. Fonseca V, Rostenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus. JAMA 2000; 283:1695-1702.
[Medline Link] [CrossRef] [Context Link]
19. Tomazic J, Karner P, Vidmar L, Maticic M, Sharma PM, Janez A. Effect of metformin and rosiglitazone on lipid metabolism in HIV infected patients receiving protease inhibitor containing HAART. Acta Dermatovenerol Alp Panonica Adriat 2005; 14:99-105.
[Medline Link] [Context Link]
20. Strowig SM, Raskin P. Combination therapy using metformin or thiazolidinediones and insulin in the treatment of diabetes mellitus. Diabetes Obes Metab 2005; 7:633-641.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
21. Schwartz S, Raskin P, Fonseca V, Graveline JF. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. N Engl J Med 1998; 338:861-866.
[Medline Link] [CrossRef] [Context Link]
22. Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996; 334:952-957.
[Medline Link] [CrossRef] [Context Link]
23. Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998; 47:507-514.
[Medline Link] [Context Link]
24. Mallon PW, Unemori P, Sedwell R, Morey A, Rafferty M, Williams K, et al. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis 2005; 191:1686-1696.
[Medline Link] [CrossRef] [Context Link]
25. Mallon P, Sedwell R, Rogers G, Nolan D, Unemori P, Wand H, et al. The effects of rosiglitazone on PPARG expression in human adipose tissue is limited by continued exposure to thymidine NRTI. In: 12th Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA, 22-25 February 2005 [Abstract 41].
[Context Link]
26. Slama L, Lanoy E, Valentin MA, Bastard JP, Chermak A, Boutekatjirt A, et al. Effect of pioglitazone on HIV-1 related lipoatrophy: a randomized double-blind placebo-controlled trial (ANRS 113) with 130 patients. In: 13th Conference on Retroviruses and Opportunistic Infections. Denver, CO, USA, 5-8 February 2006; [Abstract 151LB].
[Context Link]
27. Carr A, Workman C, Smith DE, Hoy J, Hudson J, Doong N, et al. Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial. JAMA 2002; 288:207-215.
[Medline Link] [CrossRef] [Context Link]
28. John M, McKinnon EJ, James IR, Nolan DA, Herrmann SE, Moore CB, et al. Randomized, controlled, 48-week study of switching stavudine and/or protease inhibitors to combivir/abacavir to prevent or reverse lipoatrophy in HIV-infected patients. J Acquir Immune Defic Syndr 2003; 33:29-33.
[Fulltext Link] [Medline Link] [Context Link]
29. Moyle GJ, Baldwin C, Langroudi B, Mandalia S, Gazzard BG. A 48-week, randomized, open-label comparison of three abacavir-based substitution approaches in the management of dyslipidemia and peripheral lipoatrophy. J Acquir Immune Defic Syndr 2003; 33:22-28.
[Fulltext Link] [Medline Link] [Context Link]
30. McComsey GA, Paulsen DM, Lonergan JT, Hessenthaler SM, Hoppel CL, Williams VC, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS 2005; 19:15-23.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
31. Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28:1547-1554.
[Medline Link] [Context Link]
32. van Wijk JP, de Koning EJ, Martens EP, Rabelink TJ. Thiazolidinediones and blood lipids in type 2 diabetes. Arterioscler Thromb Vasc Biol 2003; 23:1744-1749.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
33. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003; 46:459-469.
[Medline Link] [Context Link]
34. Vigouroux C, Maachi M, Nguyen TH, Coussieu C, Gharakhanian S, Funahashi T, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS 2003; 17:1503-1511.
[Fulltext Link] [Medline Link] [CrossRef] [Context Link]
35. Addy CL, Gavrila A, Tsiodras S, Brodovicz K, Karchmer AW, Mantzoros CS. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab 2003; 88:627-636.
[Medline Link] [CrossRef] [Context Link]
36. Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006; 186:5-16.
[Context Link]
37. Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004; 291:1730-1737.
[Medline Link] [CrossRef] [Context Link]
|
|
| |
| |
|
|
|