| |
Prevalence of darunavir-associated mutations in samples received for routine clinical resistance testing
|
| |
| |
"....The objective of this analysis was to determine the likelihood of response to DRV by determining the prevalence of each DRV-associated mutation and the frequency of various combinations.....In more than 108,000 clinical isolates with known PI resistance submitted for RCT, The majority (68.2%) of isolates with PI resistance defined by the FDA mutation list contained no DRV-associated mutations, while 17.8% harbored 1, 8.4% harbored 2, and only 5.6% harbored 3 or more...."
Reported by Jules Levin
AR Rinehart1; G Picchio2; M-P de Bethune3; LT Bacheler4; T Pattery5; BWasikowski6; and R Falcon1
1Tibotec Therapeutics, Bridgewater, NJ, USA; 2Tibotec Inc., Yardley, PA, USA; 3Tibotec BVBA, Mechelen, Belgium; 4VircoLab, Inc, Durham, NC, USA; 5Virco BVBA, Mechelen, Belgium; and 6xLeo, Inc., Raleigh, NC, USA
Presented at the HIV-DART, Frontiers in Drug Development for Antiretroviral Therapies, 10-14 December 2006, Cancun, Mexico.
AUTHOR CONCLUSIONS
Our findings are consistent with a recent report of the low prevalence of DRV-associated mutations in 532 patients who were on failing PI-based regimens.10
7 of the 11 DRV-associated mutations occurred alone in <1% of samples and no
mutation occurred at a frequency of >7% in 108,500 clinical isolates with evidence of resistance to PIs submitted to Virco for routine clinical resistance testing.
The prevalence of 3 or more DRV-associated mutations among isolates with evidence of resistance to PIs was approximately 6%.
These data suggest that the co-existence of 3 or more DRV-associated mutations at baseline, which appears to confer a diminished virologic response to DRV/r, is infrequent even in clinical isolates with evidence of resistance to PIs.
Abstract
Background: Darunavir (DRV) is a potent protease inhibitor (PI) with demonstrated activity in PI-resistant virus. Patients in the POWER 1, 2, and 3 studies whose viruses contained 3 or more of the protease mutations V11I, V32I, L33F, I47V, I50V, I54L/M, G73S, L76V, I84V, or L89V (DRV-associated mutations) at baseline had a diminished virologic response to DRV co-administered with ritonavir (DRV/r) 600/100mg bid. These patientsf viruses also had a median of 10 PI resistance-associated mutations (IAS-USA October 2005).
The objective of this analysis was to determine the likelihood of response to DRV by determining the prevalence of each DRV-associated mutation and the frequency of various combinations in a large number of samples received for routine clinical resistance testing (RCT).
Methods: Approximately 208,000 samples were submitted to Virco for RCT from July 1998 to June 2006. Samples were defined as PI-resistant if there was any change at amino acid positions 30, 32, 36, 46, 47, 48, 50, 53, 54, 73, 82, 84, 88, or 90 (FDA mutation list) or if the predicted fold change in IC50 for any PI was greater than the respective vircoTYPE lower clinical cut-off (vT LCCO; Virco BVBA).
Results:
108,500 samples and 91,900 samples, respectively, met the definition of having PI resistance according to the FDA mutation list or vT LCCO.
The majority (68.2%) of isolates with PI resistance defined by the FDA mutation list contained no DRV-associated mutations, while 17.8% harbored 1, 8.4% harbored 2, and only 5.6% harbored 3 or more.
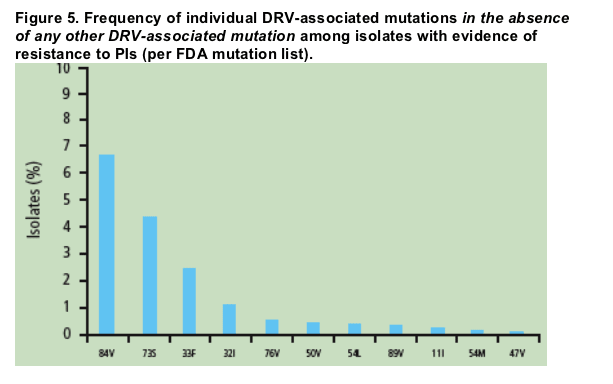
The DRV-associated mutations that occurred most frequently in the absence of any other DRV-associated mutation were I84V (6.7% of samples), G73S (4.4%), L33F (2.6%), and V32I (1.2%). The other DRV-associated mutations occurred in isolation at a frequency of <1%.
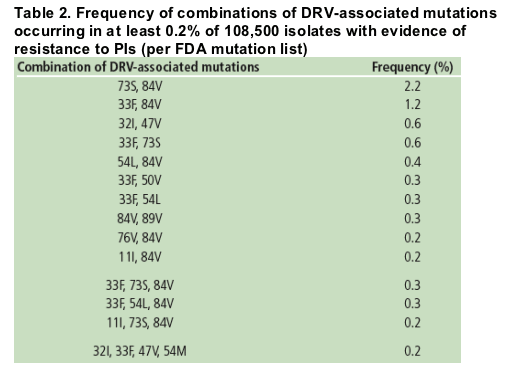
The most frequently occurring combinations of 2, 3, and 4 DRV-associated mutations were L33F+I84V (1.2% of samples), L33F+G73S+I84V (0.3%), and V32I+L33F+I47V+I54V (0.2%), respectively.
All other combinations occurred less frequently. Similar results were observed in samples with PI resistance defined by the vT LCCO.
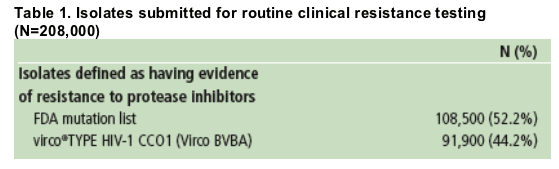
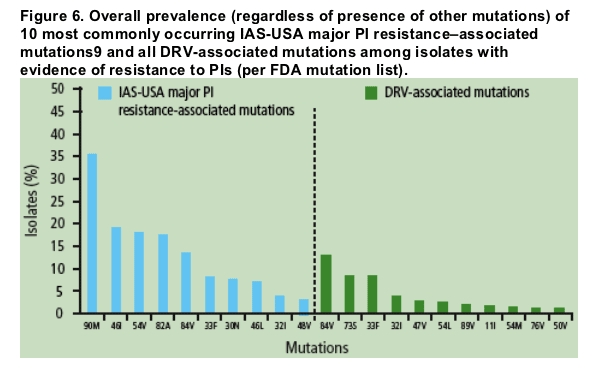
By way of comparison, the most frequently occurring IAS PI resistance-associated mutations were L90M (39.2%), L10I (29.7%), M46I (21.1%), I54V (20.7%), and V82A (17.9%).
Conclusion: In more than 108,000 clinical isolates with known PI resistance submitted for RCT, 7 of 11 DRV-associated mutations occurred alone in <1% of samples and no mutation occurred at a frequency of >7%.
The prevalence of isolates harboring 3 or more DRV-associated mutations was approximately 6%. These data suggest that the co-existence of 3 or more DRV-associated mutations at baseline, which may confer a diminished virologic
response to DRV/r, is rare even in patients with PI-resistant virus.
Data in the poster body have been updated since the abstract was originally
submitted.
INTRODUCTION
Darunavir [DRV; TMC114 (PREZISTATM)] is a protease inhibitor (PI) that has significant in vitro antiretroviral (ARV) activity against both wild-type virus and multidrug resistant HIV-1 strains.1
DRV co-administered with low-dose ritonavir (DRV/r) has been approved recently in the US, Canada, Russia, and Argentina at a recommended dose of 600/100mg bid for the treatment of HIV infection in ARV treatment-experienced adult patients.
In the POWER 1 and 2 studies and the POWER 3 analysis (safety and efficacy results presented previously),2-4 the number of DRV-associated mutations at baseline (V11I, V32I, L33F, I47V, I50V, I54L or M, G73S, L76V, I84V, or L89V) was a strong predictor of virologic response to DRV/r 600/100mg bid
- 54% of patients with 2 or fewer DRV-associated mutations at baseline achieved HIV RNA <50 copies/mL atWeek 48.
- 73% of patients in the POWER studies had viruses with 2 or fewer DRV-associated mutations.
- Viruses in these patients also had a median of 7-8 PI resistance-associated mutations (IAS USA October 20055).4
The objective of this analysis was to determine the likelihood of response to DRV by determining the prevalence of each DRV-associated mutation and the frequency of various combinations of mutations in a large number of samples with evidence of PI resistance received for routine clinical resistance testing at Virco. Samples from clinical trials are excluded from this analysis.
METHODS
Analysis was conducted in October 2006 on samples submitted through June 2006
Samples submitted to Virco for routine clinical resistance testing were defined as having evidence of PI resistance by two different methods:
- (1) Any change at amino acid positions 30, 32, 36, 46, 47, 48, 50, 53, 54, 73, 82, 84, 88, or 90 (FDA mutation list)6
- (2) Predicted fold change (FC) in IC50 for any PI greater than the respective FC representing the lower clinical cutoff value (CCO1) in the vircoTYPE HIV-1 (Virco BVBA)7
- Indinavir/r FC>10.6
- Nelfinavir FC>1.3
- Saquinavir/r FC>7.1
- Lopinavir/r FC>9.7
- Atazanavir FC>2.2
- Tipranavir/r FC>1.2
- Darunavir/r FC>3.4
- Amprenavir/r FC>1.2
- Ritonavir FC>2.4
DRV-associated mutations8 were defined as:
- V11I, V32I, L33F, I47V, I50V, I54L or M, G73S, L76V, I84V, or L89V
IAS-USA PI resistance-associated mutations9 were defined as:
- L10C/F/I/R/V, V11I, K20I/L/M/R/T/V, L24I, D30N, V32I, L33F/I, E34Q, M36I/L/V, M46I/L, I47A/V, G48V, I50L/V, F53L/Y, I54A/L/M/S/T/V, I64L/M/V, A71V/T, G73A/C/S/T, L76V, V77I, V82A/F/I/L/S/T, I84A/C/V, N88D/S, L89V, L90M, I93M
We analyzed the following patterns of resistance among all 208,000 samples
submitted for routine clinical resistance testing:
- Time trends in PI resistance according to the FDA mutation list or phenotypic CCO1
- Time trends in prevalence of individual IAS-USA PI resistance-associated mutations
- Number of DRV-associated mutations per isolate
We analyzed the following patterns of resistance among samples with evidence of PI resistance according to the FDA mutation list
- Number of DRV-associated mutations or IAS-USA PI resistance-associated mutations per isolate
- Frequency of individual DRV-associated mutations and IAS-USA PI resistance-associated mutations
- Most frequently occurring combinations of DRV-associated mutations
Unless otherwise noted, data are presented for isolates meeting the definition of evidence of PI resistance according to the FDA mutation list
Results
From July 1998 to June 2006, approximately 208,000 samples were submitted to
Virco for routine clinical testing.
Among these samples, evidence of PI resistance has been declining over time since 1999 (Figure 1).
Among isolates with evidence of PI resistance, the prevalence of most IAS-USA major PI resistance-associated mutations has remained fairly constant over time, with the exception of changes in 33F and 90M (Figure 2).
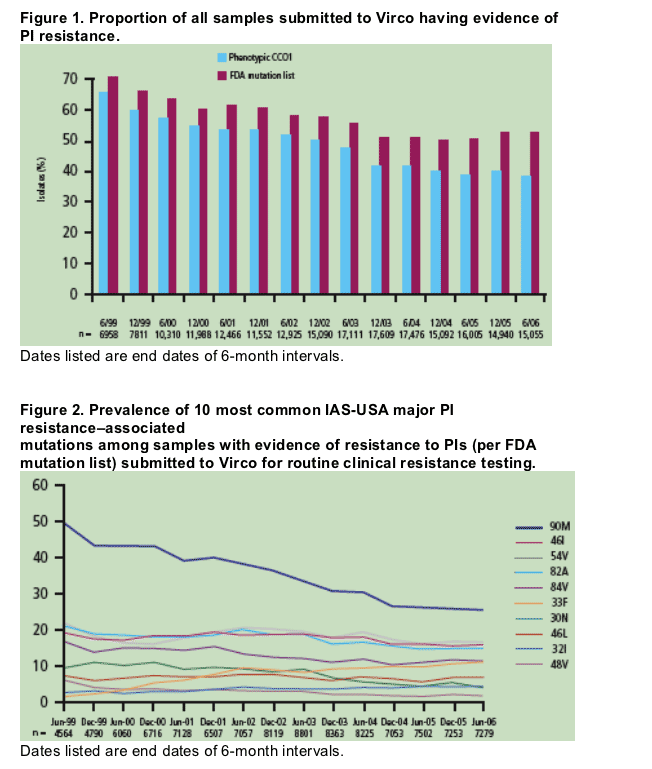
More samples met the definition of having evidence of PI resistance according to the
FDA mutation list than by phenotypic CCO1 criteria (Table 1).
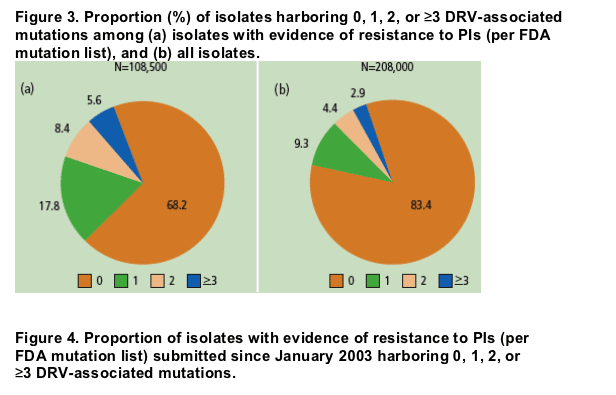
The majority (>68%) of isolates submitted since 1998 harbored no DRV-associated mutations (Figure 3).
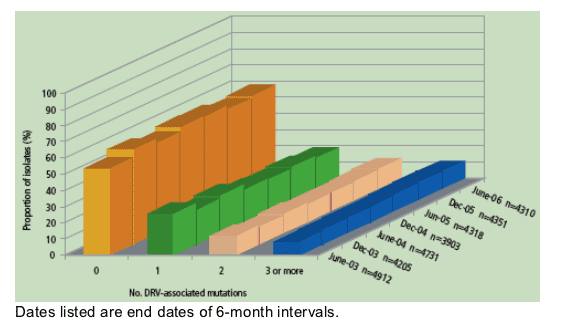
- Figure 4 shows trends from 2003 to 2006 in the prevalence of DRV-associated
mutations among samples with evidence of resistance to PIs.
Among isolates with evidence of resistance to PIs, the DRV-associated mutations that occurred most frequently in the absence of any other DRV-associated mutation were I84V (6.7% of samples), G73S (4.4%), L33F (2.6%), and V32I (1.2%) (Figure 5).
Irrespective of the presence of other mutations, DRV-associated mutations occurred much less frequently among isolates with evidence of resistance to PIs than did IASUSA major PI resistance-associated mutations (Figure 6).
Among isolates with evidence of resistance to PIs, the most frequently occurring
combinations of 2, 3, and 4 DRV-associated mutations were G73S+I84V (2.2%
of samples), L33F+G73S+I84V (0.3%), and V32I+L33F+I47V+I54M (0.2%),
respectively (Table 2).
References
1. De Meyer S, et al. Antimicrob Agents Chemother 2005;49:2314-21.
2. Bellos N, et al. 44th Annual Meeting of the Infectious Diseases Society of America, October 12-15, 2006, Toronto, Canada. Poster 958.
3. Saag M, et al. 44th Annual Meeting of the Infectious Diseases Society of America, October 12-15, 2006, Toronto, Canada. Poster 957.
4. Cohen C, et al. 44th Annual Meeting of the Infectious Diseases Society of America, October 12-15, 2006, Toronto, Canada. Oral Presentation 688.
5. Johnson VA, et al. Top HIV Med 2005;13:125-31.
6. PREZISTA prescribing information. Available at http://www.tibotectherapeutics.com/PREZISTA_pi.pdf.
7. Picchio G, et al. 46th Annual International Conference on Antimicrobial Agents and Chemotherapy, September 27-30, 2006, San Francisco, California. Poster H-999.
8. De Meyer S, et al. XV International HIV Drug ResistanceWorkshop, June 13 17, 2006. Oral Presentation and Poster 73.
9. Johnson VA, et al. Top HIV Med 2006;14:125-30.
10. Loveday C, et al. Eighth Congress on DrugTherapy in HIV Infection,November 12-16, 2006,Glasgow,UK.Abstract PL2-2.12-
|
|
| |
| |
|
|
|