| |
TMC114/r Metabolic Changes (lipids/glucose) in Healthy Volunteers Compared to Reyataz/r
|
| |
| |
Reported by Jules Levin
HIV DART: Frontiers in Drug Development for Antiretroviral Therapies; Cancun, Mexico; December 10-14, 2006.
"Similar changes in metabolic parameters of darunavir (TMC114) and atazanavir, each coadministered with low-dose ritonavir in healthy volunteers (TMC114-C159)"
F Tomaka,1 E Lefebvre,1 V Sekar,1 B Van Baelen,2
T Vangeneugden,2 A Vandevoorde,2 T Duvauchelle,3 D Miralles2
1Tibotec Inc., Yardley, USA; 2Tibotec BVBA, Mechelen, Belgium;
3SGS aster, Paris, France
OBJECTIVES
Primary objective
-- to assess the effects of RTV-boosted darunavir (DRV/r) qd on lipid and glucose metabolism compared with those of RTV-boosted atazanavir (ATV/r) qd
Secondary objective
-- to evaluate the short-term safety and tolerability of DRV/r compared with ATV/r
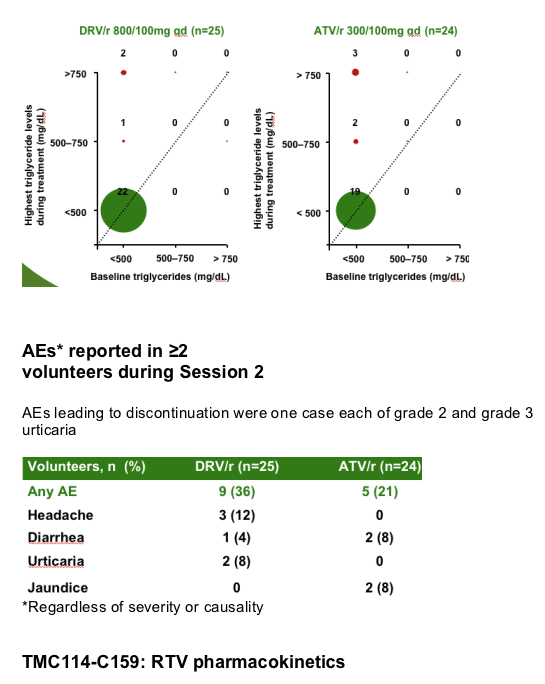
Methods: In total, 49 HIV-negative healthy male volunteers with screening lipids within normal ranges were enrolled. In Session 1, all volunteers received RTV 100mg qd for 7 days. In Session 2, volunteers received either DRV/r 800/100mg qd (Treatment A; n=25) or ATV/r 300/100mg qd (Treatment B; n=24) for 21 days. Lipid and glucose measurements were made under fasting conditions. Mean changes in laboratory values at Day 28 were calculated using Day 7 (post-RTV only) as a reference. Short-term safety and tolerability were evaluated.
AUTHOR CONCLUSIONS
Addition of DRV (TMC114) or ATV (Reyataz) to low-dose RTV resulted in small and similar changes in lipid and glucose parameters.
Both treatments were well tolerated with few adverse events.
No grade 3 or 4 lipid or glucose elevations were reported during DRV/r or ATV/r treatment.
Low-dose RTV alone resulted in rapid increases in triglycerides in HIV-negative healthy volunteers.
Administration of ATV resulted in significant increases in exposure to RTV.
TMC114-C159: trial design
Setting and testing:
--inpatient unit
medication was taken under fed conditions; meals were standardized for protein, fat and carbohydrate content
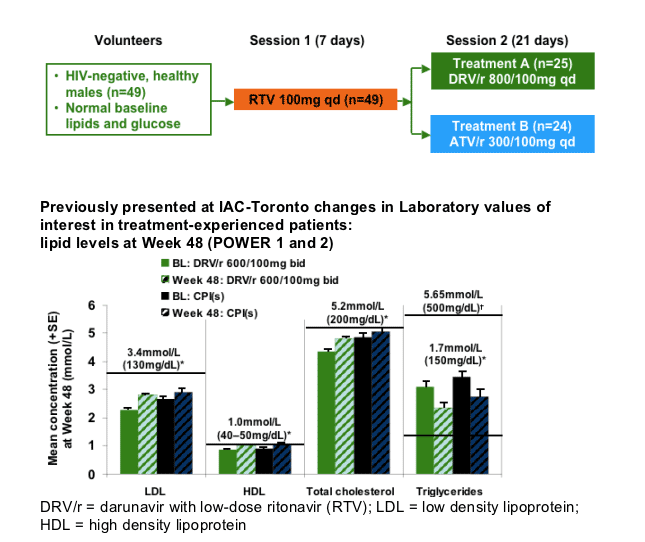
*Normal lipid levels and very high triglyceride levels taken from the Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection,
Evaluation, and Treatment of High Blood Cholesterol in Adults (JAMA.2001;285:2486-97)
(Lazzarin A, et al. 16th IAC 2006. Abstract TUAB0104)
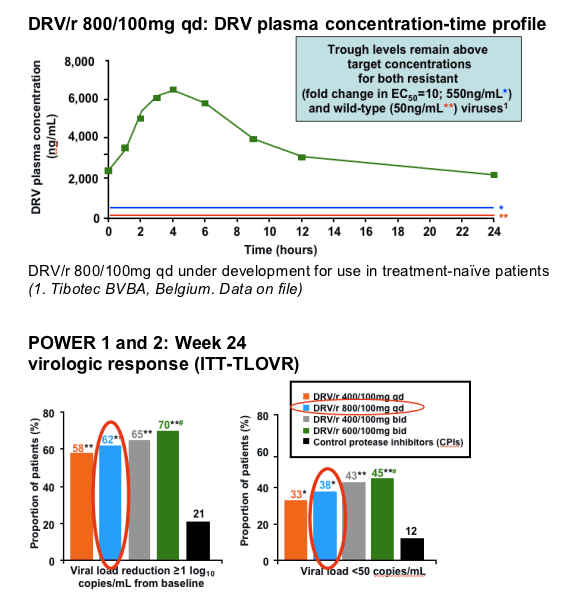
ITT-TLOVR = intent-to-treat, time to loss of virologic response
*p<0.05 in POWER 11 and 22 versus CPI(s)
**p<0.001 in POWER 11 and 22 versus CPI(s)
#p<0.05 in POWER 2 versus 400/100mg qd2
(1. Katlama C, et al. 3rd IAS 2005. Abstract WeOaLB0102
2. Wilkin T, et al. 45th ICAAC, 2005. Abstract H-413)
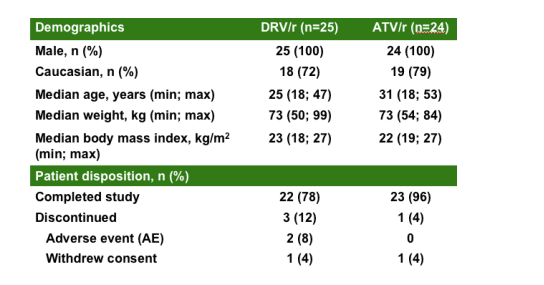
TMC114-C159: demographics
and patient disposition in the healthy volunteers study of metabolic changes:
100% men; 25 recd DRV/r, 24 recd ATV/r; 72-79% Caucasian; median age=25 for DRV/r, 31 for ATV/r; median weight (kg)= 73 for DRV/r, 73 for ATV/r; median body mass index (kg/m2)=23 for DRV/r, 22 for ATV/r;
PATIENT DISPOSITION
Completed study: 22/25 (78%) in DRCV/r, 23/24 (96%) in ATV/r.
Discontinued: 3 (12%) in DRV/r, 1 (4%) in ATV/r
Adverse events: 2 (85) in DRV/r, 1 (4%) in ATV/r
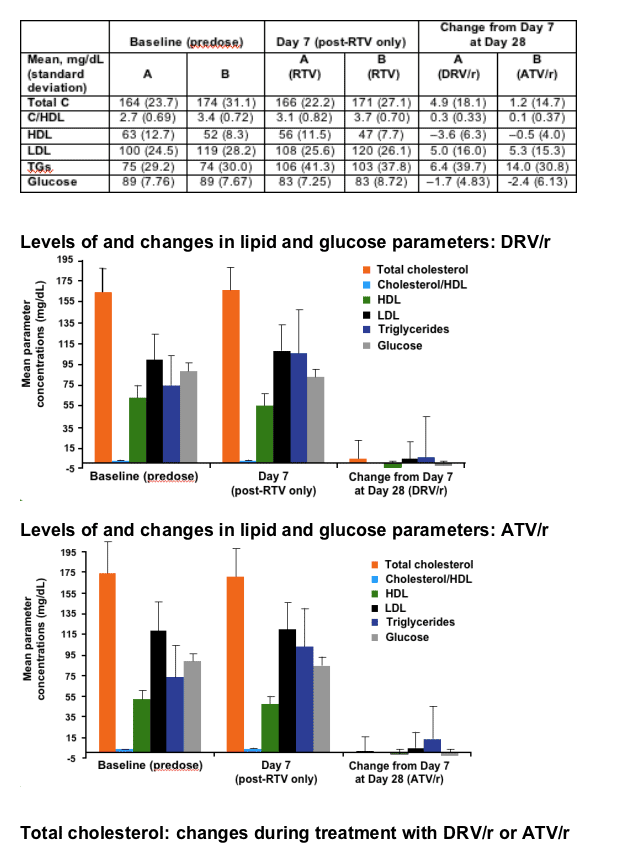
RESULTS
After 7 days of RTV treatment, triglycerides (TGs) increased by 30mg/dL and total cholesterol (C) decreased by 1mg/dL. No grade 3 or 4 lipid and glucose elevations were reported during Session 2.
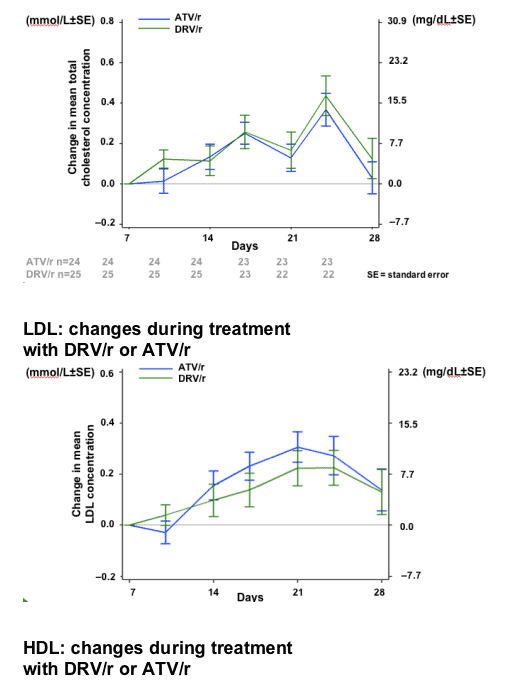
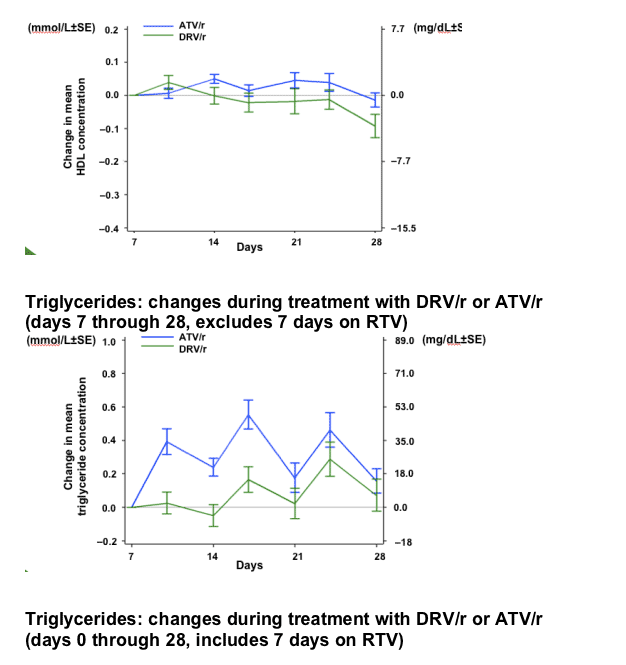
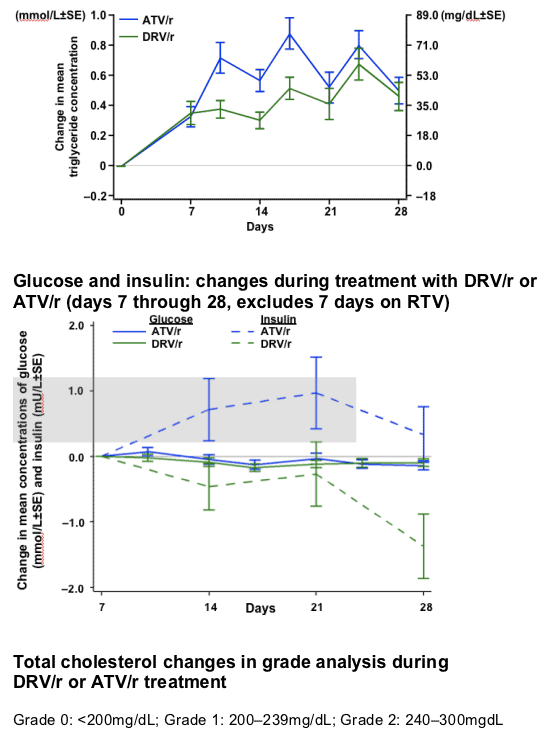
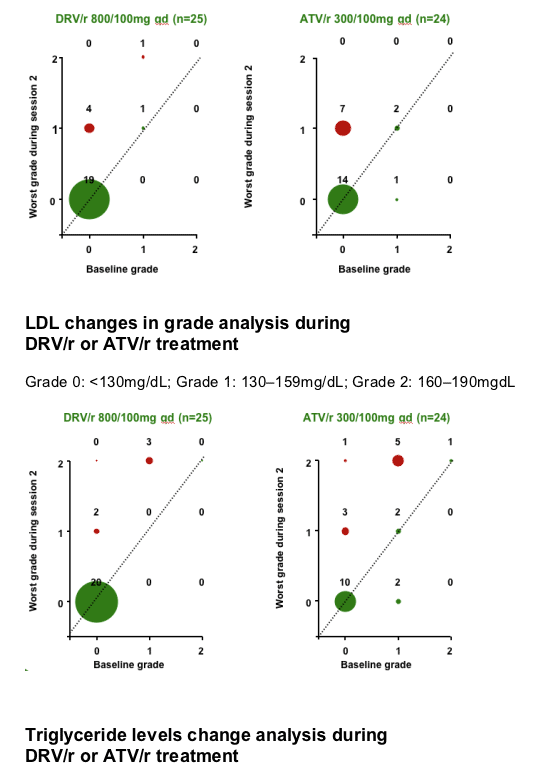
-10.gif)
|
|
| |
| |
|
|
|