 |
 |
 |
| |
Phase II Study of the Safety and Efficacy of Vicriviroc (VCV) in HIV+ Treatment-Experienced Subjects
|
| |
| |
ACTG 5211
Reported by Jules Levin
XVI Intl AIDS Conference, Toronto, Aug 2006
Presented in an oral presentation by Roy Gulick, MD
Cornell University
for the A5211 Protocol Team
A5211: Study Objectives
Primary Objective:
-- To evaluate the virologic activity of 3 doses of vicriviroc (VCV) at 14 days
Main Secondary Objectives:
At weeks 24 and 48:
-- To assess safety/tolerability of VCV
-- To evaluate virologic and immunologic activity (VCV with optimized ART)
-- To assess co-receptor switching
Study Population
-- ART-experienced adults
-- HIV RNA >5000 copies/ml on a ritonavir (RTV)-containing regimen
-- R5-only phenotype (Monogram co-receptor tropism assay)
--Stratified by:
enfuvirtide use
Baseline CD4 < or >50 cells/μL
Study Design
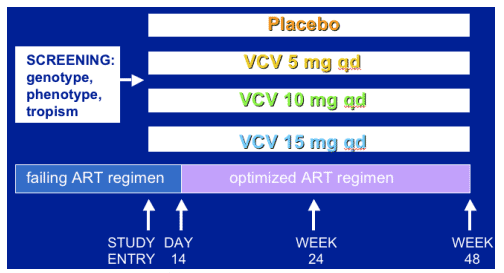
-- All regimens include 100-800 mg RTV
-- Study powered to detect >0.7 log HIV RNA difference at day 14
-- Cross-over options (Step 2) following virologic failure (after wk 16)
BASELINE CHARACTERISTICS
- 118 subjects enrolled
- median age 46
- 92% men, 8% women
- 20% black, 12% Hispanic, 66% white, 2% other
- Median HIV RNA 36380 cps/ml
- Median CD4 cell count 146 cells/μL
- 33% were ENF-experienced
- 100% had R5-tropic virus at screening
A5211: Study Monitoring Committee (SMC) Review
10/6/05: VCV 5 mg dose recommended to be stopped (and increase to 15 mg) because of:
-- trend for increased co-receptor switches
-- trend for suboptimal virologic responses
-- decision to stop lowest-dose VCV arm on Schering-sponsored rx-na´ve study
A5211: Tropism
At screening: 118 (100%) R5-only
At study entry: 102 (86%) R5-only, 12 (10%) dual/mixed, 4 (4%) missing
On initial study rx (n=106), 13 subjects changed tropism: 1 placebo, 7 VCV 5 mg, 3 VCV 10 mg, 2 VCV 15 mg
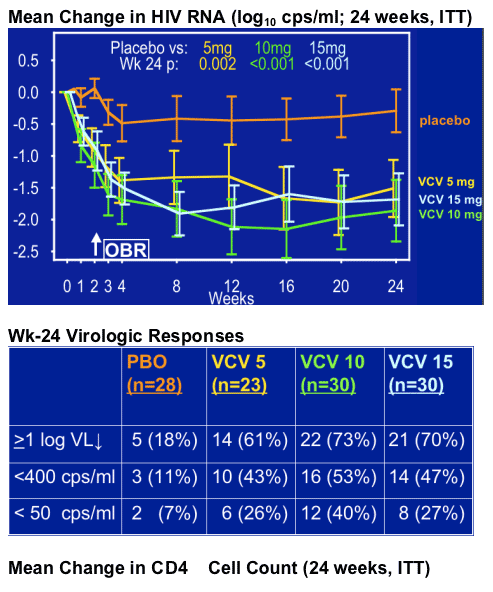
Virologic response (HIV RNA log cps/ml at week 24; VCV 10 + VCV 15 mg arms):
-- R5-tropic at entry (n=71): -1.83
-- Dual/mixed tropic at entry (n=10): -0.77
(p=.01)
Adverse Events
No significant difference for grade 3 or 4 adverse events among 4 arms (pairwise comparisons, p>0.6)
No seizures reported
5 subjects randomized to VCV developed malignancies:
-- 1 on VCV 15 mg with a hx of Hodgkin's disease (HD) developed non-Hodgkin's lymphoma (NHL)
-- 1 on VCV 10 mg with a hx of HD developed recurrent HD
--1 on VCV 15 mg developed gastric adenocarcinoma
-- 1 on VCV 5 mg developed NHL
--1 on VCV 5 mg developed HD
2 subjects randomized to PBO developed malignancies:
-- 1 subject on PBO developed multiple cutaneous squamous cell carcinomas
-- 1 subject on PBO X 7 months, who then took VCV 10 mg X 3 months, discontinued VCV for lack of response, and 1 month later developed localized perianal squamous cell carcinoma
Author Conclusions
In rx-experienced pts:
-- VCV (5, 10, 15 mg with RTV) demonstrated potent 14-day virologic suppression
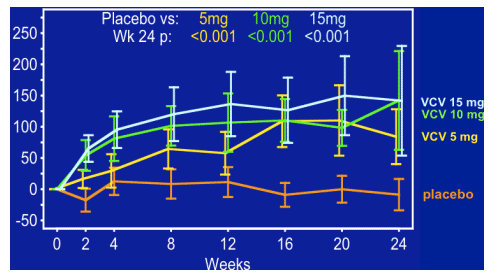
--Following optimization of background ART, VCV (10, 15 mg with RTV) demonstrated sustained antiretroviral activity over >24 weeks.
Subjects with dual/mixed-tropism had a reduced virologic response.
VCV was generally well tolerated.
The relationship of VCV to malignancy is uncertain.
|
| |
|
 |
 |
|
|