 |
 |
 |
| |
New Drugs for HCV & HBV
|
| |
| |
Reported by Jules Levin
The 2nd Intl Coinfection HIV and Hepatitis Workshop ended today in Amsterdam (Jan 12-14, 2005) and today was the best of the sessions. There were very interesting talks on
"Screening and Management for HCC" by Jessup Llovet (Mt Sinai School of Medicine, NYC)
"Liver Transplantation" in coinfection by Jose Miro, (University of Barcelona, Spain)
"PK Interaction" of HIV drugs, HCV drugs, and drugs used with liver transplantation by David Burger (Radboud University Nijmegen, The Netherlands)
"Incidence of Steatosis in a Cohort of HIV-HCV Coinfected Patients: results of a retrospective histologic study" by Raffaele Bruno (Italy)
"Assessment of Fibrosis Progression in Patients Dually Infected by HIV & HCV" by Philippe Bonnard (Paris, France)
"New Candidates (therapy) for the Treatment of Hepatitis", Marion Peters (Univ of California, SF)
Miro reported a review of studies and collected data on liver transplantation in coinfected patients finding similar responses to transplantation in coinfected compared to non-HIV+ individuals. But pre-transplant and post-transplant survival was less in HIV+ individuals, thus leading to a discussion that HIV perhaps ought to receive priority on the waiting lists for transplantation. Bruno's study report found the HIV is associated with causing steatosis (fatty liver). This led me to comment to the group that fatty liver is a much overlooked problem in HIV, that fatty liver can be caused by ART, and that fatty liver will develop into a major concern for HIV+ individuals. In Bonnard's study 2 liver biopsies were performed 4 years apart in the same study individuals and he found that HCV progressed alarmingly fast in coinfected patients. Finally, Marion Peters presented a very good review of new drugs for HCV & HBV, which is the subject of this report. Forthcoming reports will report on the important findings of the other studies mentioned above.
"New candidates for the treatment of Hepatitis B and C"
Marion Peters MD, University of California, San Francisco, USA 2005
HBV Agents currently in development
A number of agents are currently in development
Phase III
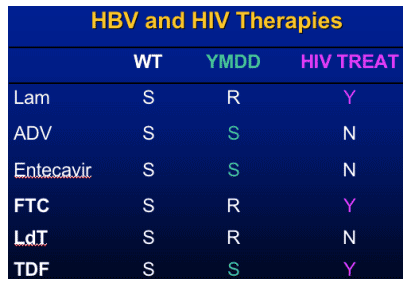
- Emtricitabine- 19% resistance YMDD
- telbivudine
- tenofovir (FDA approved for HIV emtricitabine (FTC) and TDF).
- peginterferon alfa-2b (Pegasys is approved for HBV therapy)
(entecavir was approved in 2005 for HBV therapy)
Phase II
- pradefovir
- clevudine
- valtorcitabine
Telbivudine- LdT
Specific inhibitor of HBV polymerase
- Not active against HIV or other viruses
Favorable toxicology for long-term treatment:
- Nonmutagenic, non-carcinogenic, nonteratogenic, no mitochondrial toxicity
Once daily oral dosing indicated by PK
- Consistent absorption, no food effect
Well tolerated in all clinical trials to date
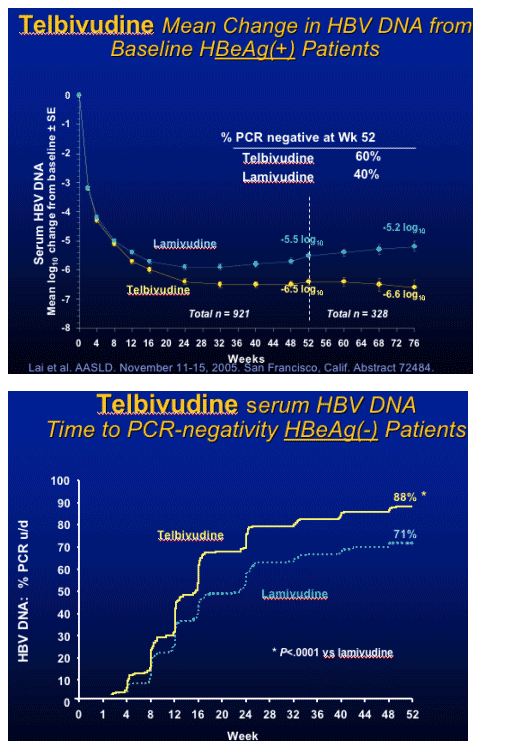
note from Jules Levin: This concept-that early viral response - dictates long-term viral response is associated with HIV therapy and other HBV & HCV drugs/therapies.
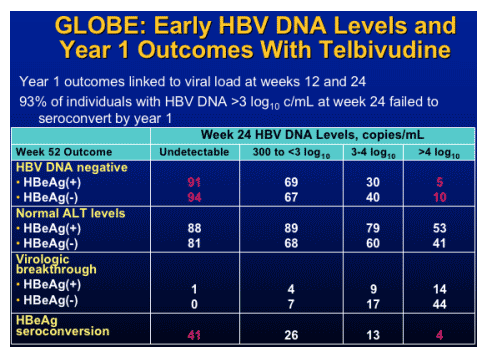
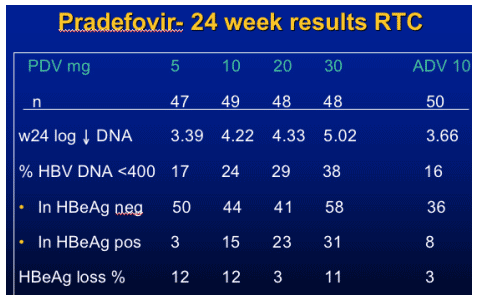
Pradefovir for HBV
- Pradefovir mesylate, a PMEA prodrug
- Metabolized by cytochrome P450 3A4 (not by GI esterases)
- Higher PMEA concentration in liver, lower concentration in kidney
- Potentially lower risk of nephrotoxicity than with adefovir
- Pradefovir demonstrated safety, HBV antiviral activity in phase 1 studies
- Current study evaluated efficacy, pharmacokinetics, and safety of pradefovir
at different doses vs adefovir in Asian patients 70% HBeAg +, genotype B/C
- No serious adverse events related to study drug
- No ALT flares or nephrotoxicity observed in any group
- Adverse events mostly similar between group
- Higher frequency of headache, gastrointestinal symptoms with pradefovir
- No dose effect
- Most mild, self-resolving
- 3 treatment discontinuations with pradefovir
- 2 possibly related to study drug
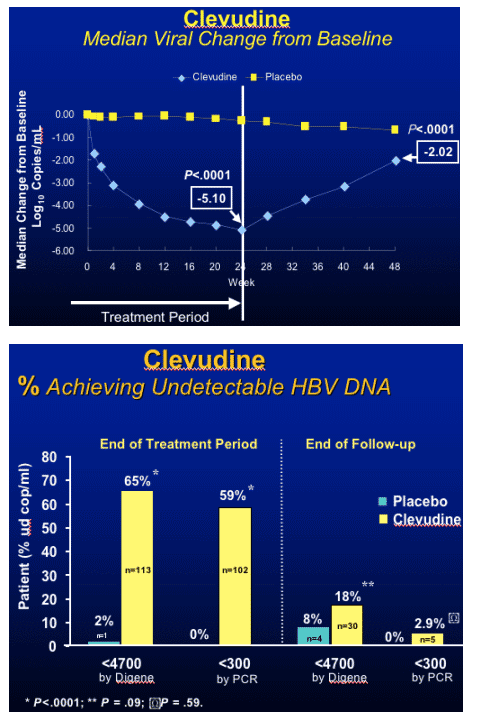
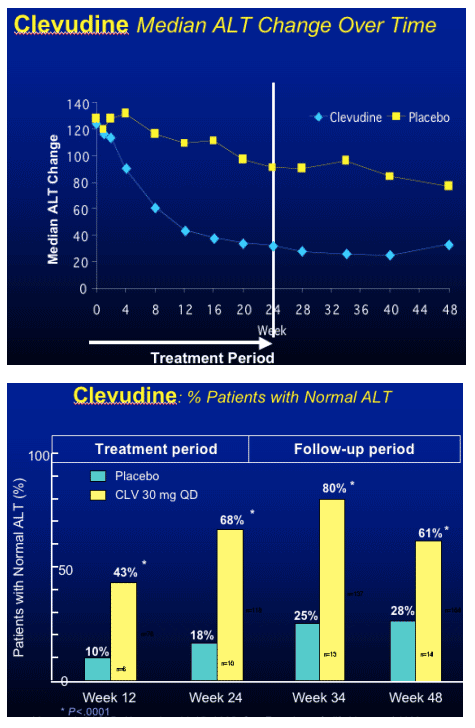
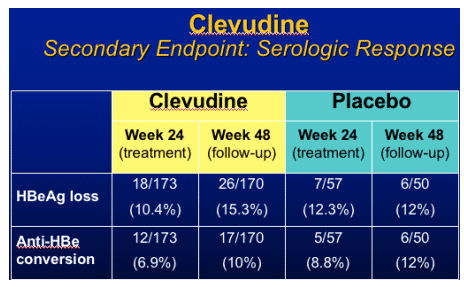
Clevudine (CLV)
HBeAg(+) Study Design
- RCT (randomized clinical trial) in HBeAg(+) chronic HBV patients
- Multicenter study at 33 sites in Korea
- Treatment with clevudine 30 mg QD or placebo for 24 weeks
- Clevudine 30 mg : Placebo = 3 : 1
- Follow-up for 24 weeks after cessation of dosing
Yoo et al. AASLD. November 11-15, 2005. San Francisco, Calif. Abstract 64169
Summary HBV
Telbivudine
- More potent than lamivudine
- Less resistance than lamivudine
Clevudine - promising for the future
Pradefovir - more liver specific
Early viral suppression
- Critical to viral response
- Minimizes viral resistance
HCV-neutralizing Antibodies
Polyclonal preparations1,2
- Inhibit or prevent infection in chimps
- Studies in man disappointing to date
Monoclonal antibodies3
- Anti-E2 human monoclonal XTL Phase 1/2
- Mild HCV RNA suppression with daily dosing
Vaccine derived anti-E1E24
- Neutralizing antibody
- In vitro inhibition of CD81 and VSV pseudovirions
VSV, vesicular stomatitis virus. 1Davis et al. Liver Transpl. 2005;11:941-949.
2Willems et al. J Hepatol. 2002;36(suppl 1):32. 3Schiano et al. AASLD. October 29-November 2, 2004; Boston, Mass. Poster 1275. 4DiBisceglie et al. Hepatology. 2005;42:750A.
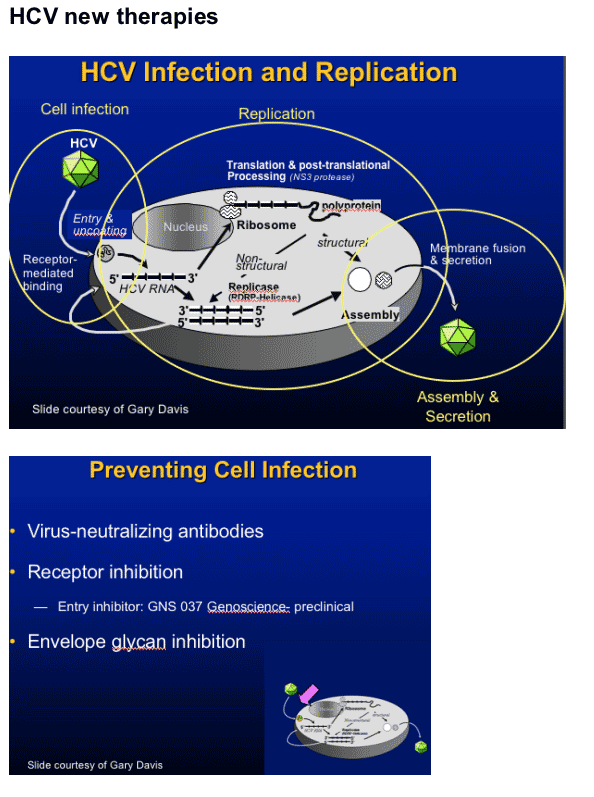
N-glycans in envelope glycoprotein (E1E2) are essential for protein folding, secretion/assembly, antigenicity, receptor binding, and cell entry
Mutations in E2 eliminate infectivity
- E2N2, E2N4 by blocking cell entry1
Imino sugars inhibit _-glucosidases and prevent proper glycosylation of viral envelope proteins; may inhibit secretion and infectivity of viruses2
1Goffard et al. J Virol. 2005;79:8400-8409.2Zitzmann et al. Proc Natl Acad Sci U S A. 1999;96:11878-11882. Mehta et al. FEBS Lett. 1998;430:17-22.
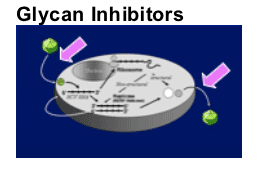
Replication Inhibitors
Translation
Polyprotein processing
- Protease inhibition
Replicase function
- Polymerase inhibition
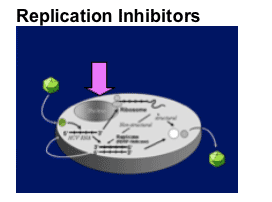
Translation
- Ribozymes
- Antisense oligos
- RNA interference
Polyprotein processing
- Protease inhibition
Replicase function
- Polymerase inhibition
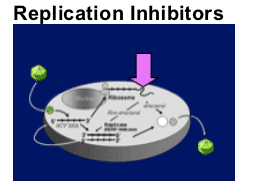
Translation
Polyprotein processing
- Protease inhibition
Replicase function
- Polymerase inhibition
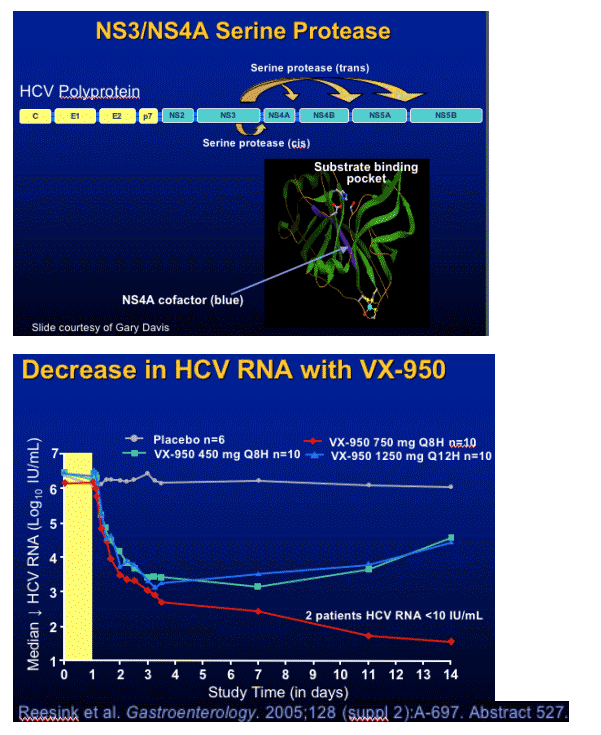
VX-950 Viral Variants
During 14 Days of Dosing
Mutations (amino acid substitution) in breakthrough and plateau subjects observed in 14-day isolates:
- A156T/V - 466-fold decrease sensitivity in vitro
- V36X - 3.5-fold
- T54S/A - 1.5 to 12-fold
- R155X in subtype 1a only - 46-fold
- 36/156 - 781-fold
After cessation of dosing, mutations persisted but the proportion of wild-type virus increased in most patients
Sarrazin et al. AASLD. November 11-14, 2005. San Francisco, Calif. Abstract 72562.
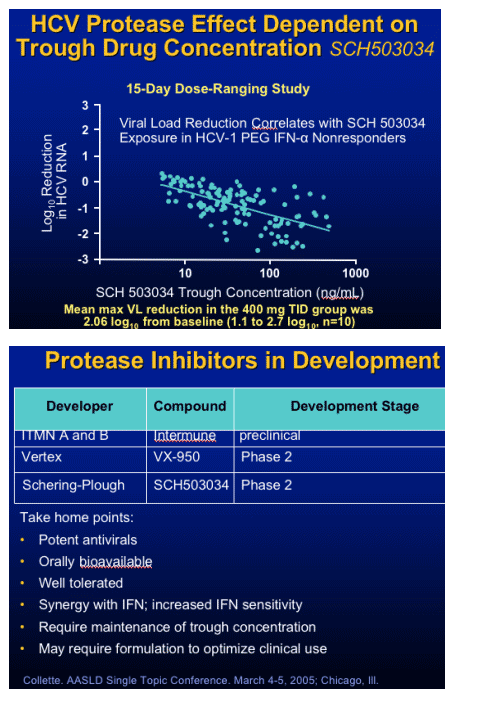
Protease Inhibitor: SCH 503034
Monotherapy
- RTC in 61 NR to Peg IFN in HCV patients
- Dosages: 100mg bid to 400 tid vs placebo
- Dose response in HCV RNA
- Best response 400 mg TID group:
2.06 log10 from baseline
Followed with combination with PegIFN
- one log decrease HCV RNA
- HCV RNA undetectable 4/10 combo, none: Peg IFN alone
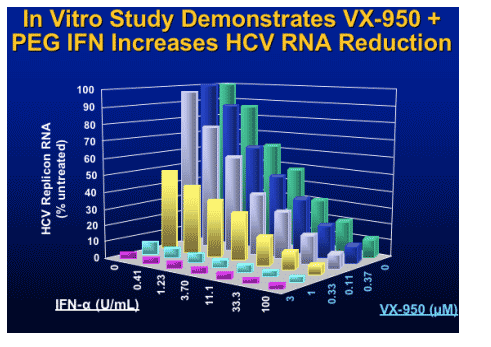
Translation
Polyprotein processing
- Protease inhibition
Replicase function
- Polymerase inhibition
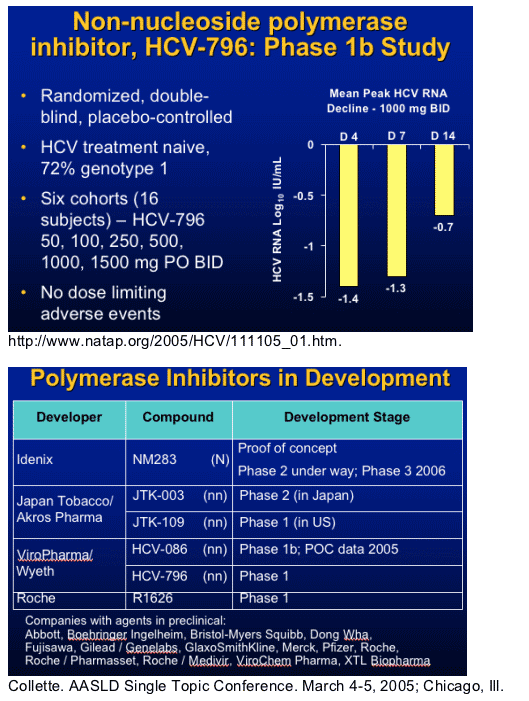
HCV RNA Polymerase
- Replicase
- Enzyme responsible for synthesis of negative RNA strand template and positive strand progeny
- Several potential sites for inhibition

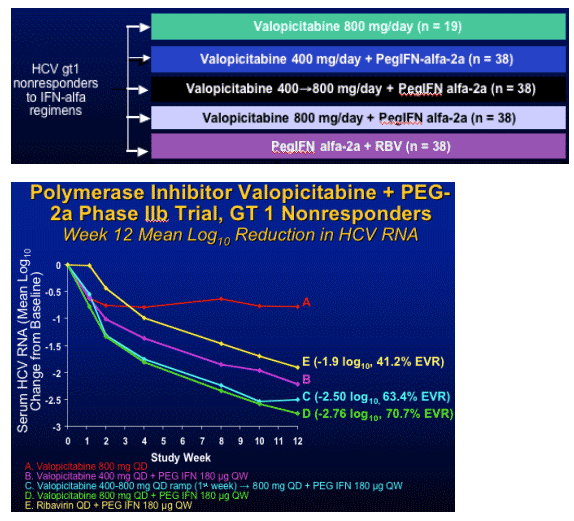
Ribonucleoside Analogue Prodrug: Valopicitabine
Multicenter (22), RCT in HCV gt1 nonresponders
HCV RNA response criteria at Weeks 4, 12, 24
- Patients required to have HCV RNA drop ≥ 0.5 log at Week 4,
>/= 1.0 log at Week 12, and > 2.0 log at Week 24 to continue treatment
- Early termination if viral response criteria not met
Primary efficacy endpoint: SVR 6 months after last dose of study medicine
Other Immune Modulators
Albuferon- Human Genome Sciences IFN-alfa fused to HAS Phase 2 studies: q 2w-
- EVR 52%; HCV RNA neg w 24 35%
CPG 0101
- TLR9 recognition by synthetic oligodeoxynucleotide with CpG motif
- Induces DC to produce cytokines for TH1 response
- Dose finding 4w Phase 1 study once or twice weekly
- Induces IFN response genes: IP-10, IFN-alfa, 2,5 OAS
- Decreased HCV RNA
Issues for New HCV Therapies
Development of combination therapies
- Different classes of agents
HCV resistance testing may be required
- Clinical role
Adherence
- 80-80-80 will not be sufficient
- >95% is probably necessary
Targets for Future HCV Therapy
Conclusions
IFN will probably remain the foundation of antiviral therapy for HCV in the near future
There are several potential new targets for therapy
A few agents have reached human testing, but results are preliminary (first generation)
More effective therapy in man is likely to involve combinations of drugs that utilize different strategies for virus inhibition
|
|
| |
| |
|
 |
 |
|
|