 |
 |
 |
| |
Risk of Hepatotoxicity in Virologically Suppressed HIV+ Patients switching to Nevirapine According to Gender & CD4 Count
|
| |
| |
Reported by Jules Levin
8th Intl Workshop on Adverse Drug Reactions and Lipodystrophy in HIV
San Francisco, Sept 24-26, 2006-09-25
Background: There is an increased risk of hepatotoxicity in ART-na´ve patients starting a nevirapine-containing combination ART regimen with high CD4 counts. It is not known whether this high risk also applies to virologically suppressed patients.
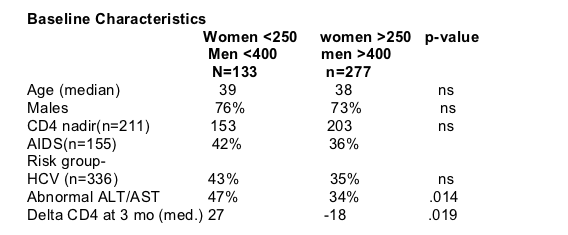
This was a meta-analysis of all randomized studies in which virologically suppressed patients were switched to a NVP HAART regimen and have a 3 month followup. CD4 was classified as high (>400 in men/>250 in women) or low. The main study endpoints were death and hepatotoxicity defined as elevation of ALT or AST above 200 if normal at baseline or 3 or more fold increase if abnormal at baseline within the first 3 months. Mortality, sympotomatic hepatitis, and rash were also evaluated. The 4 studies are: QDLIuita (JAIDS 2001, Ruiz L); BOEHRINGER (JAIDS 2003, Montaner J); NEFA (NEJM 2003, Martinez, E); PREVIHNE II (JAIDS 2004, Knobel H). There were 133 patients with (women <250, men <400 CD4s): 50 NEFA, 80 Previne 2, 19 qdLiuita, BOEHRINGER 35. There were 277 patients with (women >250, men >400): 105 NEFA, 103 previne 2, 65 qdLiuita, 35 BOEHRINGER; total: 410 patients.
Author conclusions:
According to the resultsof this meta-analysis, we were unable to detect a higher risk for hepatotoxicity or rash in virologically suppressed patients switching to NVP (nevirapine) containing HAART.
The results of this meta-analysis are in accordance with those from a recent analysis from the EuroSida Study (presented at Toronto IAC Aug 2006). Taken together, these data do not support a role of gender & CD4 count on the risk of developing hypersensitivity to NVP in virologically suppressed patients switching to NVP-containing HAART.
In the table immediately below there was no difference in the incidence of events between the two patients groups high vs low CD4 counts. Death was <1 in high CD4 group vs 1% in low CD4 group; hepatotoxicity or death was 8% in both groups; hepatotoxicty or rash was 20% in low CD4 group vs 17% in high CD4 group; rash or death was 14% in low CD4 group vs 10% in high CD4 group; and symptomatic hepatitis or death was 1% in low CD4 group vs 1% in high CD4 group.
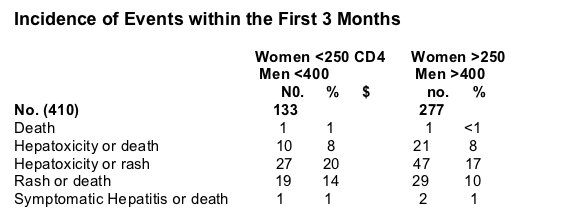
None of the following variables were selected by multivariate models as associated with an increased risk of hepatitis:
Baseline CD4/gender
HCV
Age
Baseline transaminases. OR=0.125 (-0.02, 0.3) P=0.08
Incidence of hepatoxicity at any time: Kaplan-Meier p=0.8. The program book said differences in hepatoxicity of 8% or greater would have been detected with a power of 80% and <0.05 if existed.
|
| |
|
 |
 |
|
|