 |
 |
 |
| |
Bone Loss 62% in HIV+
|
| |
| |
CROI 2007 Bones
Reported by Jules Levin, NATAP
....assess HIV+ patients at baseline for bone mineral density .....identify people with risk factors- prevent fractures
.....osteoporosis appears to be occurring at an earlier age on HIV+
In the SUN Study below, 10% of HIV+ had osteoporosis compared to 1% from normal HIV-negatives. 52% of HIV+ had osteopenia (average age 41 yrs), 62% had low bone mineral density. A group from Mass General found "these data demonstrate that BMD is abnormal among HIV infected women, in association with low weight, reduced lean mass, reduced androgen levels, and abnormal menstrual function". An ACTG group from ATG 384 found bone mineral density declined 16 weeks after starting ART, low testosterone was associated with decline, and they suggest HIV, immune and viral changes may be associated with change, and low baseline CD4 was associated with decline. A group from Italy looked at bone metaolism in IV+ children and adolescents. They concluded "The current data confirm the presence of an altered bone metabolism in HIV-infected children and adolescents....high bone resorption rate seems to be the result of a profound modification of the factors regulating osteoclastogenesis..... in vitro evidence indicates a role of HIV infection and antiviral treatment......Our patients were all on long-term HAART, and thus the high concentration of RANKL observed may be in part due to the use of antiviral drugs".
Factors Associated with Low Bone Mineral Density in a Large Cohort of HIV-infected US Adults: Baseline Results from the SUN Study
Et Overton*1, K Mondy1, T Bush2, L Conley2, E Kojic3, K Henry4, J Hammer5, K Wood6, K Lichtenstein7, J Brooks2, and SUN Study Investigators
1Washington Univ Sch of Med, St Louis, MO, US; 2CDC, Atlanta, GA, US; 3The Miriam Hosp, Providence, RI, US; 4HIV Prgm, Hennepin County Med Ctr, Univ of Minnesota, Minneapolis, US; 5Denver Infectious Disease Consultants, Rose Med Ctr, CO, US; 6Cerner Corp, Vienna, VA, US; and 7Univ of Colorado Hlth Sci Ctr, Denver, US
Background: Low bone mineral density (BMD) is a common metabolic complication associated with HIV infection. With increasing survival in the era of HAART, there is continued interest in the factors influencing low BMD in HIV-infected individuals.
Methods: The SUN Study prospectively follows a cohort of HIV-infected patients at clinics in Denver, Minneapolis, Providence, and St. Louis. At baseline, DEXA bone densitometry and body composition, clinical data, and fasting laboratory values were obtained on all subjects. Results were compared to persons from the National Health and Nutrition Examination Study III (NHANES) matched for age, race, gender, and BMI.
Results: Data were available for 562 matched pairs. Participants had a mean age of 41.0 years, 22% were female, and 27% were African American. Among SUN subjects, 79% were currently on HAART and 60% had an undetectable HIV viral load. Osteopenia (T-score -1.0 to -2.5) and osteoporosis (T-score ≦ -2.5) were identified in 262 (47%) and 59 (11%) subjects, respectively. In univariate analyses, low BMD (T-score ≦ -1.0) was associated with older age, male gender, unemployment, lower body mass index, higher visceral to subcutaneous fat ratio, duration since HIV diagnosis and any stavudine use (all p ≦0.01). Osteoporosis alone was associated with older age, non-white race, lower body mass index, current tobacco use, unemployment, lower nadir CD4 cell count, and longer duration since HIV diagnosis (all p ≦0.05). In multivariate analyses, low BMD was associated with older age, male gender, lower body mass index, unemployment, and stavudine use; osteoporosis was associated with older age, non-white race, lower body mass index, longer duration since HIV diagnosis, no resistance training, and unemployment (all p <0.05).
When compared to the NHANES cohort, the SUN Study cohort had significantly lower mean T-scores at the femoral neck (-0.79 vs -0.36, p ≦0.01). At this site, the prevalence of low BMD was higher in the SUN Study cohort (47% vs 31%, p ≦0.01).
Conclusions: In the HAART era, low BMD remains a significant complication of HIV infection, more prevalent than found in the general population. In this large cohort of HIV-infected persons, the majority of whom are virologically controlled, the ramifications of low BMD will become a concern as they age. HIV- and ART-related factors that may affect BMD loss warrant longitudinal study.
The Effects of Weight, Body Composition, and Testosterone on Bone Mineral Density in
HIV-Infected Women
Sara E. Dolan, RN, PhD(c), Sara Carpenter, BA, and Steven Grinspoon, MD
Program in Nutritional Metabolism, Massachusetts General Hospital and Harvard Medical School, Boston, MA USA
Author Conclusions: These data demonstrate that BMD is abnormal among HIV infected women, in association with low weight, reduced lean mass, reduced androgen levels, and abnormal menstrual function.
Background: The simultaneous effects of weight, body composition, and androgen deficiency on bone mineral density (BMD) have not been described in HIV-infected women.
Methods: This study examined the effects of reduced androgen levels, changes in weight, body composition and menstrual dysfunction on bone mineral density among 152 HIV infected women characterized by normal weight (>90% IBW, n=124) and low weight (< 90% IBW, n=28) compared to 100 non HIV-infected control subjects. Free testosterone was determined by equilibrium dialysis. Bone density was determined by dual energy X-ray absorptiometry (DEXA). Abdominal fat was determined by single slice CT-scan at L4. A menstrual history was obtained.
Results: A significant difference was seen in BMD between groups at the lumbar
spine (0.92 + 0.02 g/cm2 vs. 1.01 + 0.01 g/cm2 vs. 1.07 + 0.01 g/cm2, p <0.0001), total hip (0.84 + 0.03 g/cm2 vs. 0.94 + 0.01 g/cm2 vs. 0.98 + 0.01 g/cm2 p <0.0001), and femoral neck (0.73 + 0.03 g/cm2 vs. 0.83 + 0.01 g/cm2 vs. 0.87 + 0.01 g/cm2 p <0.0001) [HIV-infected low weight, HIV-infected normal weight and non HIV-infected control subjects, respectively, for each comparison]. Among the HIV infected subjects, lumbar BMD correlated with IBW (r=0.37, p = <0.0001), total body lean (r=0.43, p = <0.0001), total body fat (r=0.35, p = <0.0001), and SAT (r=0.41, p = <0.0001) but not VAT (r=0.07, p=0.417). Clinical risk factors for osteopenia and osteoporosis in the HIV population included low free testosterone (< 1.1 pg/ml [lower limit of the normal
range of free testosterone for women])(3.8 pmol/L) (p = 0.0007), low weight (p =
0.014), and oligiomenorrhea (p = 0.0006).
Demographic & Clinical Characteristics
Average age is 39-41. Over 50% are current smokers among HIV+. Free testosterone is 6.2 in HIV+ Low weight, 7.1 in HIV+ ormal weight, and 9.6 in HIV-negative controls. BONE DENSITY MEASURES are worse for HIV+ low weight compared to HIV+ normal weight, and Bone density measures are worse for HIV+ normal weight compared to HIV-negative controls.
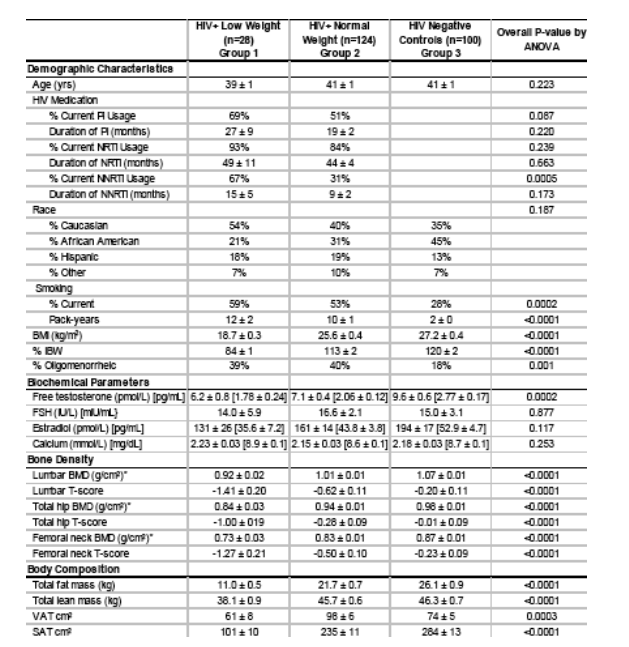
Comparison of Bone Mineral Density by Free Testosterone
Bone mineral denity is worse at lumbar spine, total hip, and femoral neck for HIV+ with low testosterone. HIV+ with low weight are worse than HIV+ with normal weight.
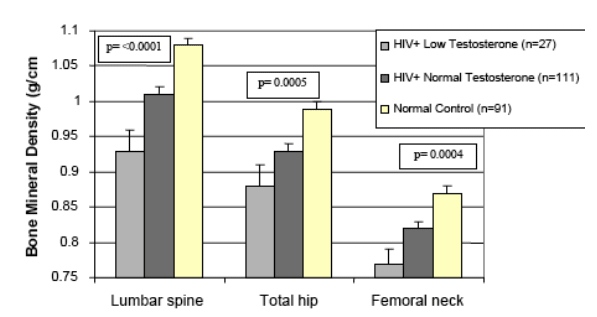
Risk Factors for Osteopenia and Osteoporosis
Compared to normal HIV-negative controls HIV+ with low weight, low testosterone and oligomenorrheic are more likely to have osteppenia.
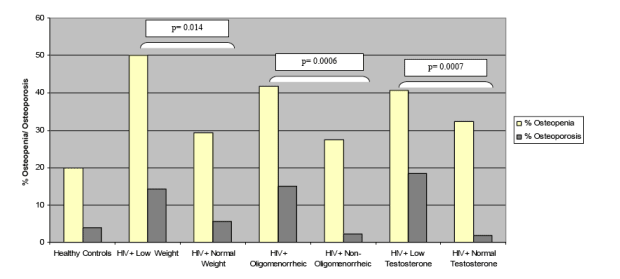
Comparison of Bone Mineral Density by Weight
HIV+ with low weight have lower BMD at lumbar spine, hip, and femoral neck. HIV+ with normal weight have lower BMD than HIV-negative women.
Alteration of Circulating Osteoimmune Factors May Be Responsible for Bone Metabolism Derangement in HIV-infected Children and Adolescents
S Mora1, V Giacomet2, I Zamproni1, L Cafarelli2, G Pattarino2, D Frasca2, G Zuccotti2, and Alessandra Vigan˜*2
1San Raffaele Sci Inst, Milan, Italy and 2L Sacco Hosp, Univ of Milan, Italy
Background: The discovery of NFkB ligand (RANKL) and osteoprotegerin (OPG) dramatically changed our understanding of the processes underlying regulation of bone resorption. RANKL and OPG potently stimulate and inhibit, respectively, osteoclast formation. Bone metabolism derangement has been described in HIV-infected children. We investigated the possible role of RANKL and OPG on bone metabolism alterations in pediatrics patients.
Methods: We studied 27 vertically HIV-infected children and adolescents (aged 4.9 to 17.3 years) on long-term HAART (70±8 months). All patients received lamivudine (3TC), stavudine (d4T), and 1 protease inhibitor (PI). Their mean CD4 number was 841±291, mean CD4% was 34±6, and HIV RNA was <50 copies/mL. As a control group, we enrolled 336 healthy children, aged 4.8 to 17.9 years. Serum concentration of bone alkaline phosphatase (BAP), RANKL, and OPG were measured by EIA assays. The concentration of NTx (a bone resorption index) was measured by ELISA assay in timed urine samples. Comparisons between HIV-infected children and healthy children subjects were performed by multivariate analyses, to correct for the confounding effect of sex and age on markersÕ concentration.
Results: Patients and control subjects did not differ for age, sex distribution, and anthropometric measurements. Patients had significantly (p <0.0001) higher concentration of BAP (128±49 U/L) and NTx (374±231 nM BCE/mM Cr), compared to healthy children (104±51 U/L, and 253±139 nM BCE/mM Cr, respectively). Serum concentration of RANKL was 2.1±1.9 pmol/L in HIV, and 0.4±0.7 pmol/L in healthy children (p <0.0001). Patients showed significantly (p <0.0001) higher concentration of OPG (10.3±2.4 U/L), compared to healthy children (5.3±1.4 U/L). The ratio of RANKL/OPG was significantly higher (p = 0.013) in HIV-infected children (0.22±0.27), compared to healthy controls (0.07±0.14).
Conclusions: The current data confirm the presence of an altered bone metabolism in HIV-infected children and adolescents. The high bone resorption rate seems to be the result of a profound modification of the factors regulating osteoclastogenesis, since the ratio between OPG and RANKL was in favor of the latter. Moreover, in vitro evidence indicates a role of HIV infection and antiviral treatment in the production of RANKL by T cells. Our patients were all on long-term HAART, and thus the high concentration of RANKL observed may be in part due to the use of antiviral drugs.
Initiation of ART Is Associated with Bone Loss Independent of the Specific ART Regimen. The Results of ACTG A5005s
Pablo Tebas*1, T Umbleja2, M Dube3, R Parker2, K Mulligan4, R Roubenoff5, G Robbins2, S Grinspoon2, and ACTG
1Univ of Pennsylvania, Philadelphia, US; 2Harvard Univ, Boston, MA, US; 3Indiana Univ, Indianapolis, US; 4Univ of California, San Francisco, US; and 5Tufts Univ, Boston, MA, US
Background: Osteopenia is very frequent among HIV-infected individuals. Both HIV infection and its treatment play a role in its development. However, longitudinal data of the effects of initiating ART on bone mineral content are very limited. The goals of this study were to measure bone mineral content among ART-na•ve individuals, to evaluate the longitudinal effects of the initiation of ART on bone, and to study the relationships of bone changes with other metabolic parameters.
Methods: In A5005s, the metabolic substudy of AIDS Clinical Trials Group (ACTG) 384, whole-body DEXA scans were performed at entry and every 16 weeks thereafter in 157 subjects. Participants were randomized to receive nelfinavir (NFV), efavirenz (EFV), or both drugs combined with open-label zidovudine (AZT) and lamivudine (3TC) or didanosine (ddI) and stavudine (4dT) (NRTI groups). Percentage change in total bone mineral content was the primary outcome variable.
Results: After the initiation of ART, there was a small but statistically significant decrease in total bone mineral content at 16 weeks (median: -0.44%, IQR -1.96 to 1.07%, p = 0.02). Total bone mineral content continued to decrease. At 48 weeks, the decrease was 1.02% (IQR -3.63 to 1.24%, p <0.01), while at 96 weeks, the decrease was 2.25% (IQR -4.75 to 0.35%, p <0.01). Even though the decline in total bone mineral content in the EFV group was slightly less using a mixed models analysis limited to participants remaining on original treatment, the difference between the NFV and EFV groups was not statistically significant (p = 0.08), nor was the effect of nucleoside reverse transcriptase inhibitor (NRTI) assignment (p = 0.60). Higher baseline CD4 cell count was associated with a slower decline in total bone mineral content after ART initiation (p = 0.002). Baseline total bone mineral content correlated weakly with HDL cholesterol (R = -0.24, p <0.01) and percentage of free testosterone (R = 0.26, p <0.01). CD4 and HIV RNA levels were not associated with baseline total bone mineral content. Correlations between changes in total bone mineral content and other metabolic parameters were generally not significant.
Conclusions: The initiation of ART was associated with a modest but statistically significant bone loss that was independent of the regimen used in our study. The amount of bone loss after ART initiation was greater in individuals with lower baseline CD4. These findings suggest that the treatment effect of ART on bone was not a direct toxic effect, but might be mediated through the antiviral or immunological changes associated with the initiation of ART.
|
| |
|
 |
 |
|
|