 |
 |
 |
| |
Four Double-DUET Analyses Give Closer Look at New NNRTI
|
| |
| |
11th European AIDS Conference
October 24-27, 2007
Madrid
Mark Mascolini
DUET-1 and DUET-2, the already published trials of the nonnucleoside (NNRTI) etravirine (TMC125) [1,2], may still have only 24 weeks of data to analyze, but DUETicians scrutinized those data from four perspectives at the European AIDS Conference in Madrid [3-6]. The principal study, a pooled 24-week efficacy analysis of DUET-1 and -2, found that 59% of people with resistance to efavirenz or nevirapine--plus resistance to protease inhibitors (PIs)--reached a viral load below 50 copies when they combined etravirine with darunavir/ritonavir [3]. A resistance analysis found that no single NNRTI mutation predicted a poor response to etravirine, but most responders had fewer than three etravirine-related mutations.
The DUET studies signed up 1200 people with at least one NNRTI mutation and at least three primary PI mutations and assigned them to etravirine or placebo plus darunavir/ritonavir and other antiretrovirals. In fact nearly two thirds of study participants had two or more NNRTI mutations and four or more PI mutations. They began their etravirine regimen with a median viral load around 65,000 copies and a median CD4 count around 100.
In a 24-week time-to-loss-of-virologic-response analysis, 59% of patients randomized to etravirine had a viral load under 50 copies, compared with 41% randomized to placebo, a highly significant difference (P < 0.0001). Among people using enfuvirtide for the first time, 67% taking etravirine and 62% taking placebo reached a 24-week load under 50 copies, and that difference lacked statistical significance (P = 0.427). But more people in the etravirine group than the placebo group had resistance to darunavir when the study began. So when DUET statisticians adjusted the 24-week response for starting susceptibility to darunavir in people taking enfuvirtide for the first time, the 50-copy response difference between etravirine and placebo (73% versus 62%) did reach statistical significance (P < 0.05).
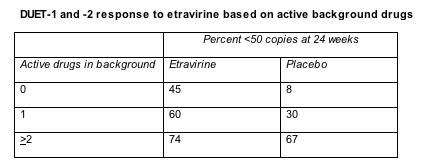
Among people starting no active drugs besides etravirine or starting one active drug with etravirine, 24-week response rates were much better with the new NNRTI than with placebo (Table). But that response difference between etravirine and placebo almost disappeared in people who started two or more active drugs with etravirine.
When DUET researchers analyzed 24-week responses according to starting viral load (<30,000, 30,000 to 99,000, or >100,000 copies) or starting CD4 count (<50, 50 to 99, or >100 cells), people taking etravirine consistently had better responses than people taking placebo. Etravirine also consistently outperformed placebo regardless of how many darunavir mutations or etravirine mutations people had when they started salvage.
DUET virologists identified 13 baseline NNRTI mutations that correlated with a diminished response to etravirine: V90I, A98G, L100I, K101E/P, V106I, V179D/F, Y181C/I/V and G190A/S [4]. A large majority of DUET participants, 86%, had fewer than three of these mutations, and they had the best 24-week virologic responses. Among people with no etravirine-related mutations when DUET began, 75% had a sub-50-copy response at 24 weeks. Response rates dwindled to 60% with one etravirine mutation at baseline and to 58% with two. Among people who started etravirine with three of these mutations, only 41% had a viral load under 50 copies at 24 weeks. And among those who started with four or more of these mutations, only 25% made it under the 50-copy mark. But regardless of the number of starting etravirine mutations, the NNRTI consistently outdid placebo at 24 weeks. The fearsome K103N mutation had little effect on response to etravirine.
The proportion of patients with a new AIDS illness did not differ significantly between patients randomized to etravirine versus placebo, except when the investigators limited the analysis to people reusing or not using enfuvirtide [5]. In that case 7.2% taking placebo versus 3.1% taking etravirine had a new AIDS diagnosis (P = 0.0067). While 16% of patients taking placebo got sent to the hospital during the study, 11% taking etravirine landed in the hospital, also a significant difference (P = 0.0031).
Rash was the only side effect that troubled more people taking etravirine than placebo--about 10% versus 3% at week 2 [6]. But clinicians usually rated these rashes mild to moderate, and most rashes cleared up as etravirine therapy continued.
References
1. Valdez Madruga J, Cahn P, Grinsztejn B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:29-38
2. Lazzarin A, Campbell T, Clotet B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:39-48.
3. Katlama C, Gatell JM, Molina JM, et al. Pooled 24-week results of DUET-1 and -2:
efficacy of TMC125 in treatment-experienced HIV-1-infected patients. 11th European AIDS Conference. October 24-27, 2007. Madrid. Abstract P7.3/16.
4. Vingerhoets J, Clotet B, Peeters M, et al. Impact of baseline NNRTI mutations on the virological response to TMC125 (etravirine; ETR) in the DUET-1 and DUET-2 phase III clinical trials. 11th European AIDS Conference. October 24-27, 2007. Madrid. Abstract P7.3/05.
5. Gatell J, Beatty G, Johnson M, et al. Impact of TMC125, a next-generation NNRTI, on clinical outcomes (AIDS-defining illnesses and deaths): 24-week findings from a planned pooled analysis of the DUET studies. 11th European AIDS Conference. October 24-27, 2007. Madrid. Abstract P7.3/18.
6. Di Perri G, Girard PM, Clumeck N, et al. Pooled 24-week results of Duet-1 and -2: TMC125 (Etravirine; ETR) safety and tolerability in treatment-experienced, HIV-1-infected patients. 11th European AIDS Conference. October 24-27, 2007. Madrid. Abstract P7.3/17.
|
| |
|
 |
 |
|
|