 |
 |
 |
| |
Saquinavir/r (SQV/r) vs lopinavir/r (LPV/r) plus emtricitabine/tenofovir (FTC/TDF) as initial therapy in HIV-1 infected patients
The Gemini Study
|
| |
| |
Reported by Jules Levin
11th EACS, Oct 24-27, Madrid
Walmsley S,1 Ruxrungtham K,2 Slim J,3 Ward D,4
Larson P,5 and Raffi F6
1University of Toronto, Toronto, Canada; 2HIV-NAT, Thai Red Cross AIDS Research Centre, Bangkok, Thailand; 3St Michael's Medical Center, Newark, NJ, USA; 4Dupont Circle Physicians Group, Washington DC, USA; 5 Roche, Nutley, NJ, USA; 6University Hospital, Nantes, France.
AUTHOR CONCLUSIONS
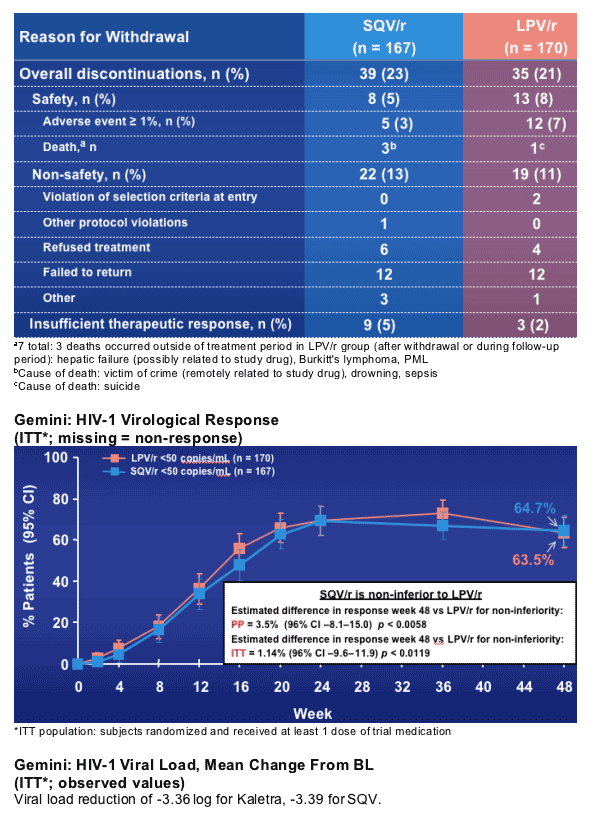
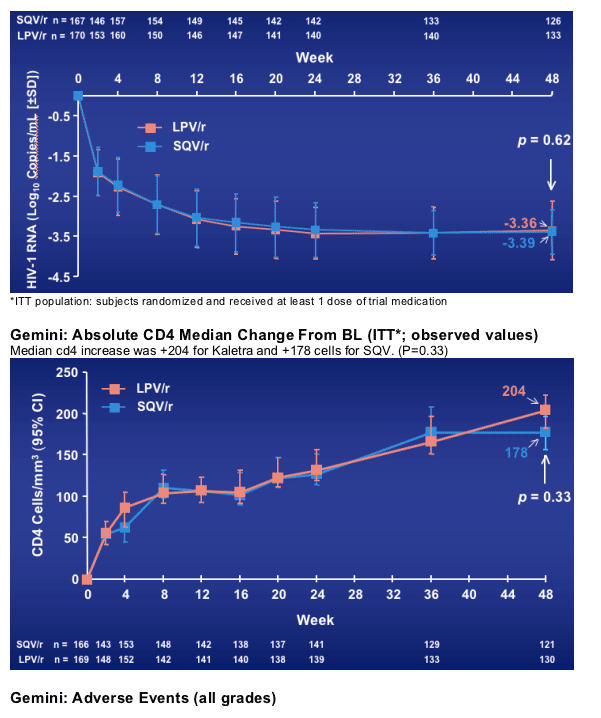
Gemini demonstrates non-inferiority of SQV/r (% subjects reaching VR <50 copies/mL) to LPV/r in the treatment of HIV-1 infected ARV-naive adults
In this study population with advanced untreated HIV disease, both regimens showed substantial CD4 cell count increases
Adverse events were reported with similar frequency and resulted in few discontinuations in either arm
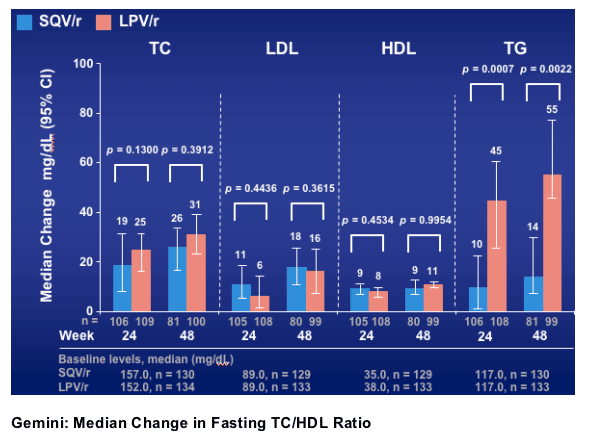
Significantly lower elevations from baseline in median triglycerides were observed with SQV/r at weeks 24 and 48
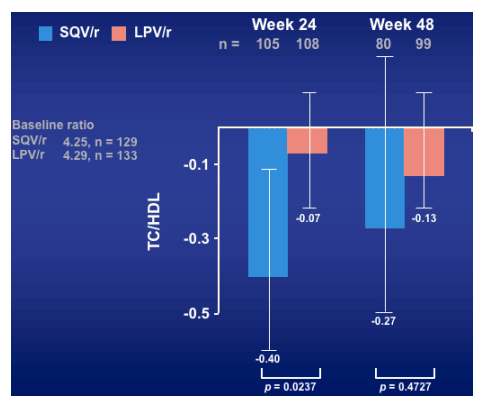
Decrease in TC/HDL ratio for SQV/r at week 24 (but not at week 48) was significantly greater than for LPV/r
Overall, these results confirm that SQV/r is an efficacious and well-tolerated PI for use in treatment-naive patients with HIV-1 infection
Gemini provides further evidence for the lack of emergence of PI resistance with failure of first-line boosted PI regimens
GEMINI Study design
The study randomizes 337 treatment-naives to saquinavir/r 1000/100 mg bid plus FTC/TDF 200/300 mg qd or Lopinavir/r 400/100 mg bid plus FTC/TDF 200/300 mg qd.
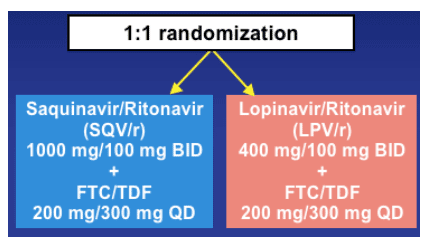
Prospective, randomized, multicenter, open-label trial
N = 337
--USA, Canada, France, and Thailand
Duration = 48 weeks
Inclusion criteria
--Treatment naive
-- CD4 count ≦350 cells/mm3
-- HIV-1 RNA >10,000 copies/mL
Exclusion criteria
--Previous treatment with antiretrovirals (≥2 weeks exposure)
--Evidence of OI or intercurrent illness
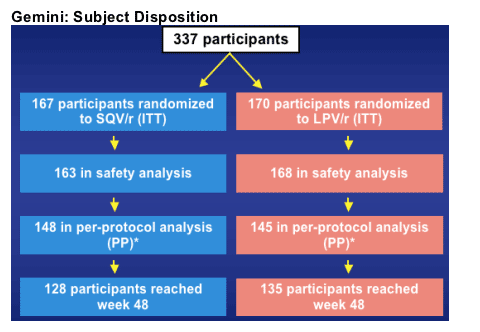
*17 participants in SQV/r and 25 in LPV/r arm excluded from PP analysis primarily because of entry criteria violations: HIV-1 ≦10,000 copies/mL, CD4 count >350 cells/mm3, and HBsAg+. Two additional participants in the SQV/r arm were excluded: 1 for use of prohibited medication and 1 for a major protocol violation.
Gemini: Study Objective and End Points
To demonstrate non-inferiority SQV/r vs LPV/r at week 48 in treatment-naive, HIV-1 infected adults
--Non-inferiority defined as lower CI threshold of _12%
Primary efficacy end point
--Patients (%) with HIV-1 RNA <50 copies/mL at week 48
Secondary efficacy and tolerability endpoints
--Time course of virologic suppression (<50 copies/mL and <400 copies/mL)
--Time course of CD4 cells/mm3 increase
--Safety as assessed by clinical and laboratory AEs, SAEs and deaths
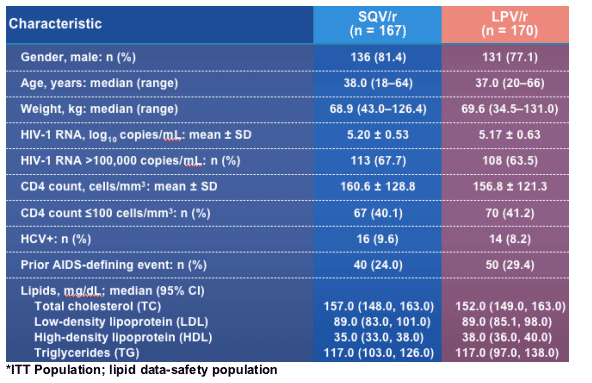
Gemini: Demographics and Baseline Characteristics*
About 80% were men. 9% HCV+. Mean HIV RNA 5.20 log for SQV and 5.17 log for Kaletra. Viral load >100,000 c/ml was 67.7% for those on SQV and 63.5% on Kaletra. Mean cd4 count was160 for SQV and 156 for Kaletra. % with cd4 less than 100 cells was 40% for SQV and 41% for Kaletra. Levels for lipids were very similar between the 2 groups at baseline: total cholesterol was 157 for SQV and 152 for Kaletra; LDL was 89 for each group; HDL was 35 for SQV and 38 for Kaletra; triglycerides were 117 for SQV and 117 for Kaletra. Prior AIDS-defining event: 24% SQV, 29% Kaletra.
Gemini: Subject Discontinuations
There were 39/167 (23%) discontinuations in the SQV group, and 35/170 (21%) for Kaletra group;
--for safety: 5% on SQV and 8% on Kaletra
--for adverse event (>/=1%): 3% on SQV, 7% on Kaletra
--death: 3 on SQV, 1 on Kaletra.
--NON-SAFETY: 13% SQV, 11% Kaletra
violation of selection criteria at entry: 0 SQV, 2 Kaletra
other protocol violations: 1 SQV, 0 Kaletra
refused treatment: 6 SQV, 4 Kaletra
failed to return: 12 in each group
other: 3 SQV, 1 Kaletra
--insufficient therapeutic response: 5% (n=9) SQV, 2% (n=3) Kaletra.
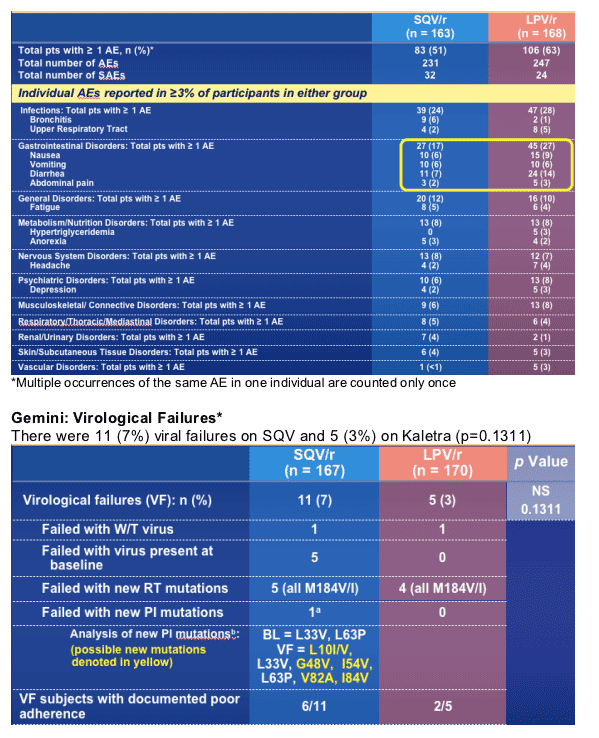
*Virological failure defined as 2 consecutive counts of HIV RNA >400 copies/mL at week 16 or after
aDocumented poor adherence; also had M184V
bNew PI mutations were described at week 24 in 2 separate subjects. On further ongoing analyses, in 1 subject, the D60E mutation was subsequently confirmed in the baseline sample; for the subject presented here, L10I/V was confirmed to be new, but baseline sample could not be amplified past codon 46
Gemini: Median Change in Fasting Lipid Levels (mg/dL)
Total cholesterol increased by 26 mg/dl from baseline to week 48 for SQV and 31 mg/dl for Kaletra. LDL increased by 18 mg/dl for SQV, 16 mg/dl for Kaletra; HDL increased by 9 mg/dl for SQV, 11 mg/dl for Kaletra; triglycerides increased by 14 mg/dl for SQV, 55 mg/dl for Kaletra (p=0.0022).
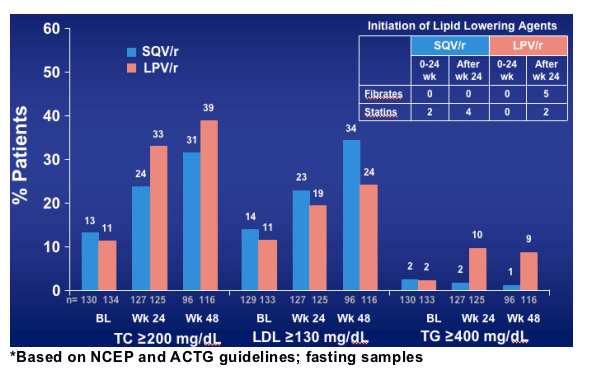
Gemini: Subjects Exceeding Lipid Levels that May Warrant Clinical Intervention*
Total chol: at baseline 13% on SQV and 11% on Kaletra had >200 mg/dl chol, and at week 48, 31% on SQV AND 39% on Kaletra had >200 mg/dl chol. For LDL, 14% on SQV and 11% on Kaletra at baseline had LDL >130 mg/dl, and after 48 weeks 34% on SQV and 24% on Kaletra had >130 mg/dl. For triglycerides, at baseline 2% in each group had >400 mg/dl, and after 48 weeks 1% on SQV and 9% on Kaletra had >400 mg/dl.
|
| |
|
 |
 |
|
|