 |
 |
 |
| |
Pooled 24-week results of DUET-1 and DUET-2: TMC125 (etravirine; ETR) safety and tolerability in treatment-experienced, HIV-1-infected patients
|
| |
| |
Reported by Jules Levin
11th EACS, Oct 24-27, 2007, Madrid
G Di Perri,1 PM Girard,2 N Clumeck,3 M Peeters,4 M Janssens,4 G De Smedt4
1University of Turin, Turin, Italy; 2Service des maladies infectieuses et tropicales, Université Pierre et Marie-Curie, Paris, France;
3Service des maladies infectieuses, Brussels, Belgium; 4Tibotec BVBA, Mechelen, Belgium
Abstract
Objectives: DUET-1 and DUET-2 are identically designed, ongoing, randomised, double-blind, placebo-controlled, Phase III trials, investigating TMC125 versus placebo in HIV-1- infected, treatment-experienced patients. The trials differ only by geographical location. We report findings from a planned, pooled analysis of safety in DUET-1 and DUET-2 when all patients had reached Week 24 or discontinued.
Methods: Patients on stable virologically-failing treatment, with documented NNRTI resistance (historical and/or at study entry) and 3 or more primary protease inhibitor (PI) mutations were randomised 1:1 to TMC125 200mg or placebo twice daily (each with darunavir/ritonavir [DRV/r], optimised NRTIs and optional enfuvirtide [ENF]). Prespecified intent-to-treat (ITT) analyses of rash, nervous system, psychiatric and hepatic adverse events (AEs) were by Fisher's exact test.
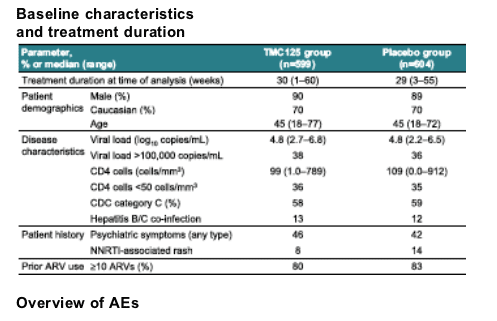
Results: 1,203 patients were treated (89.3% male; 69.8% Caucasian; 58.4% CDC category C), with median baseline viral load of 4.8 log10 copies/mL and CD4 cell count of 105 cells/mm3. Patients (n=599) received TMC125 for a median treatment duration of 30 weeks. The safety and tolerability findings are summarised in the table.
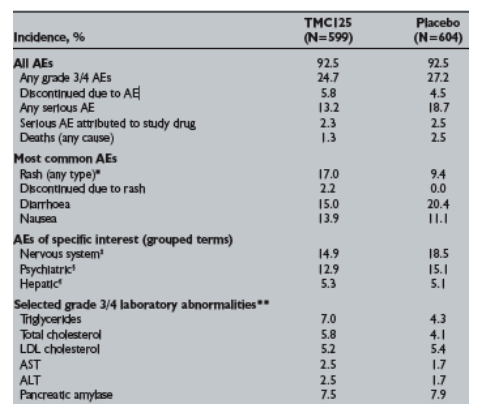
*p=0.0001. Rashes occurred more frequently with TMC125 than placebo, but were generally grade _ in severity (1.3% of patients experienced grade 3 rash). Rashes were more common in women (28.3% vs 15.8% men) and not related to CD4 cell count. Events generally started 1-2 weeks following treatment-initiation and resolved on continued treatment. p=0.0896; p=0.2803; p=0.8976; **No
consistent or clinically relevant changes in laboratory or ECG parameters were reported.
Conclusions: The incidence and severity of AEs with TMC125, including neuropsychiatric AEs, were generally similar to placebo. Rash, generally mild-to-moderate and self-limited, was the only AE to occur more frequently with TMC125 than placebo.
Please note that these data have been updated following submission of this abstract.
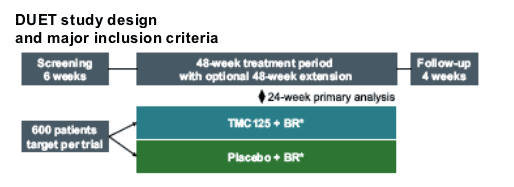
DUET 1 and 2 differed only in geographical location; pooled analysis was pre-specified.
Major inclusion criteria:
--plasma viral load >5000 HIV-1 RMA copies/ml and stable therapy for 28 weeks
--1 or more NNRTI nutations,* at screening or in documented historical genotype
--3 or more primary PI mutations at screening
Patients recruited from Thailand, Australia, Europe, and the Americas
*from extended list of NNRTI mutations.
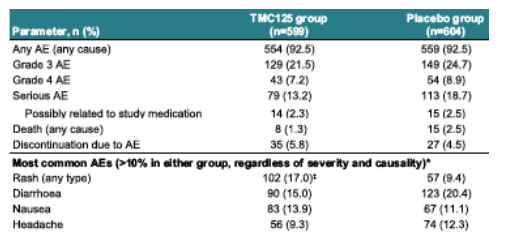
AEs leading to death were not reported in more than one patient except for pneumonia and sepsis (n=2 each) in the placebo group.
--none of the deaths in the TMC125 group were considered related to TMC125.
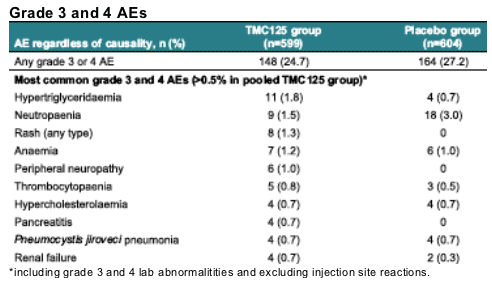
*excluding injection site reactions **p=0.0001 vs placebo (Rash)
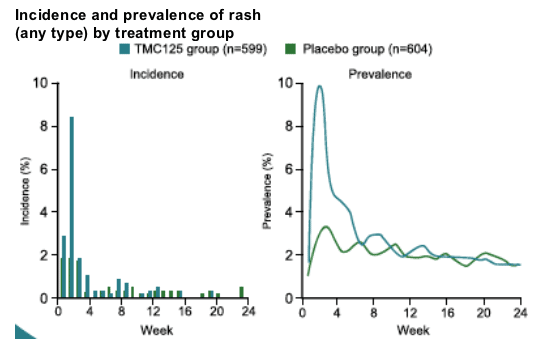
AEs of interest: rash
Overall incidence
--regardless of causality: 17% in TMC125 group vs 9.4% in placebo group (p=0.0001)
--considered at least possibly related to study medication: 12.2% vs 4.8% in the TMC125 group vs placebo groups, respectively.
In the tmc125 group
--early onset: median onset day 12
--limited duration: median duration 11 days
--low severity: usually mild-to-moderate; 1.3% grade 3 and 0% grade 4
mostly maculopapular in nature; no rashes with mucosal involvement
--infrequently led to permanent discontinuation: 2.2% of patients
most self-limiting with continued treatment
--higher incidence in women (28.3% vs 15.8% in males), but no difference in severity or discontinuations between genders
--no association with baseline cd4 cell count.
--no increased risk in patients with a history of NNRTI-related rash.
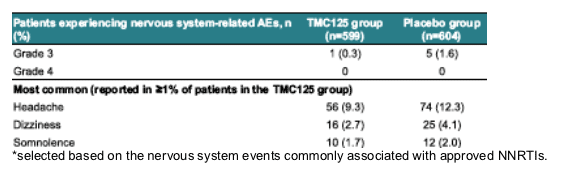
AEs of interest: nervous system*
--Similar incidence to placebo: 14.9% in TMC125 group vs 18.5% in placebo group (p=0.0896)
--low severity: mostly grade 1 or 2, with grade 3 AEs in <2% of patients in both groups and no grade 4 AEs reported.
--did not lead to discontinuation in the TMC125 group: no patients in the TMC125 group and 1% of patients in the placebo group.
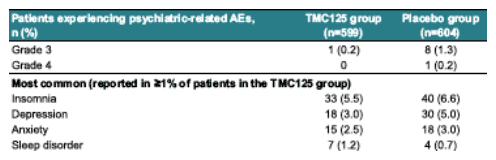
AEs of interest: psychiatric disorders
--similar incidence to placebo: 12.9% in TMC125 group (p=0.2803)
--low severity: mostly grade 1 or 2, with grade 3 AEs reported in <2% of patients in both groups and no grade 4 AEs reported in the tmc125 group.
--infrequently lead to discontinuation: one patient (0.2% in each group)
--no increased risk in patients with a history of psychiatric disorders
--anmormal dreams/nightmares in 5 patients (0.8%) in each group and no episodes of hallucinations, suicidal ideation or manic symptoms in the tmc125 group.
Hepatic AEs and laboratory
Abnormalities
Hepatic AEs
--similar incidence to placebo: 5.3% in TMC125 group vs 5.1% in placebo group
--low severity: mostly grade 1 or 2
--infrequently led to discontinuation: 0.7% of patients in both groups
--hepatitis co-infected patients: most hepatic AEs were grade 1 or 2. Grade 3 or 4 hepatic AEs reported with similar incidence in TMC125 and placebo groups (4.2% vs 4.4%, respectively)
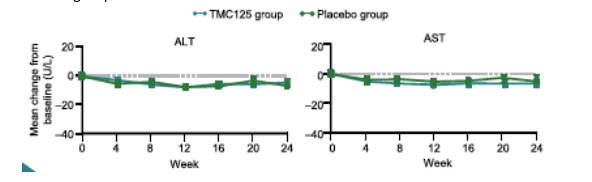
--ALT and AST lab abnormalities: incidence of grade 3 ALT and AST elevations was low (about 2% vs 1.3% with placebo) with <1% grade 4 elevations in both groups.
|
| |
|
 |
 |
|
|