 |
 |
 |
| |
Impact of baseline NNRTI mutations on the virological response to TMC125
(etravirine; ETR) in the DUET-1 and DUET-2 Phase III clinical trials
|
| |
| |
Reported by Jules Levin
11th EACS< European AIDS Conference, Oct 23-27, 2007, Madrid
J Vingerhoets,1 B Clotet,2 M Peeters,1 G Picchio,3 L Tambuyzer,1 K Cao Van,1 G De Smedt,1 B Woodfall,1 MP de Bethune1
1Tibotec, Mechelen, Belgium; 2Hospital Universitari Germans Trias i Pujol and irsiCaixa Foundation, UAB, Barcelona, Catalonia, Spain;
3Tibotec, Yardley, PA, USA
AUTHOR CONCLUSIONS
Analysis of the pooled DUET data indicated that TMC125 has a unique resistance profile
-- no single mutation was identified to have a significant impact on virological response to TMC125
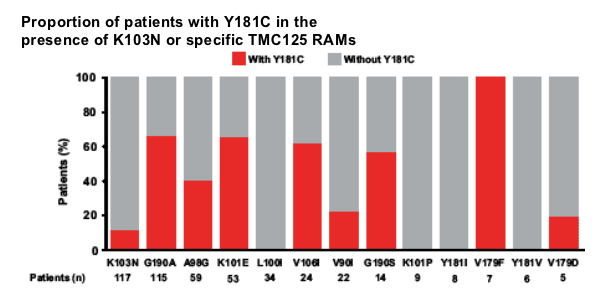
-- thirteen baseline RT mutations were associated with decreased virological response to TMC125 (TMC125 RAMs): V90I, A98G, L100I, K101E/P, V106I, V179D/F, Y181C/I/V and G190A/S.
The majority of patients (86%) had <3 TMC125 RAMs, and these patients experienced the greatest benefit with TMC125 treatment.
An increased number of baseline TMC125 RAMs was associated with a decreased virological response to TMC125.
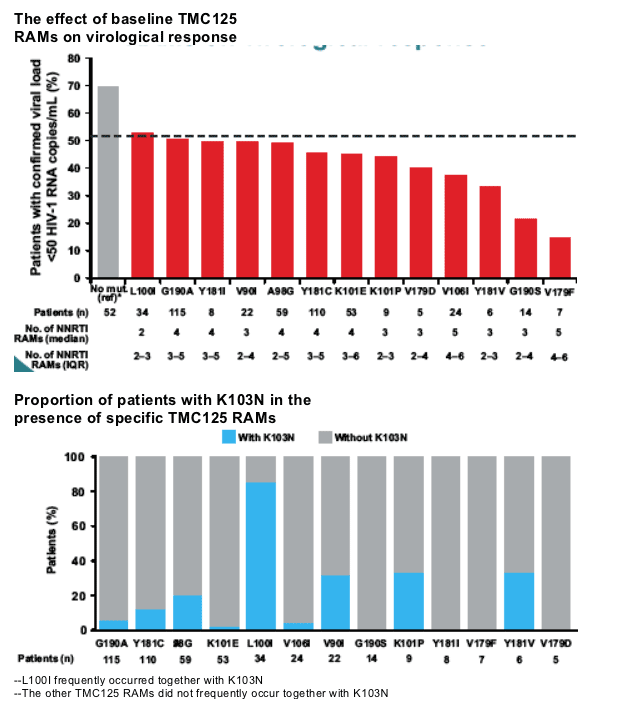
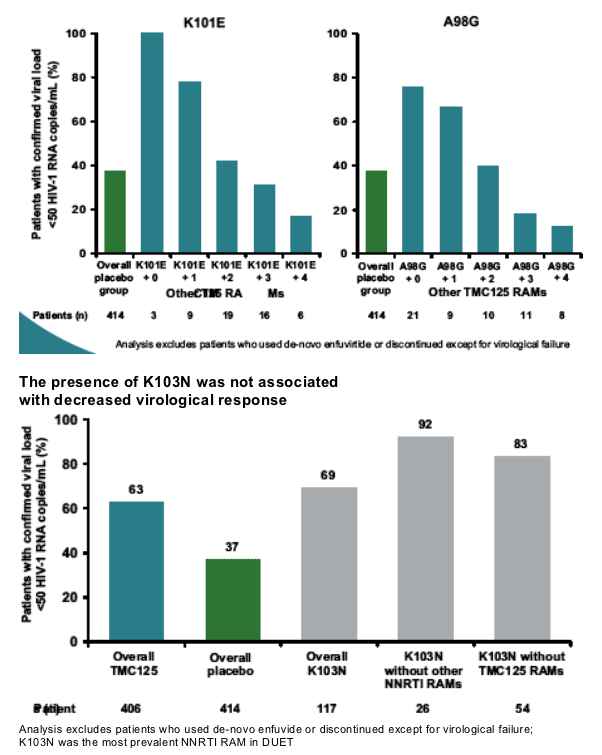
A high proportion of patients with K103N at baseline achieved a virological response (<50 copies/mL).
These data provide guidance in the interpretation of genotypic resistance data related to TMC125.
Abstract
Background: TMC125 is a next-generation NNRTI, active against wild-type and NNRTI-resistant HIV-1, with a high genetic barrier to the development of resistance. Here, we identified baseline genotypic determinants of decreased virological response to TMC125 in the Phase III, double-blind, placebo-controlled DUET trials.
Methods: Effect of baseline genotype on virological response (<50 HIV-1 RNA copies/mL) to TMC125 at Week 24 was studied in patients not using enfuvirtide de novo, and excluding discontinuations for reasons other than virological failure (n=406). Decreased virological response was defined as a response that was at
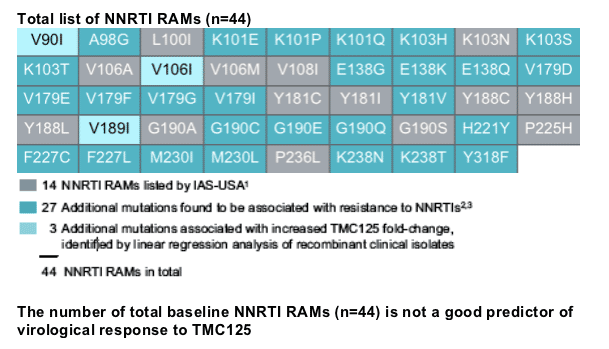
least 25% lower than that of the subgroup of patients without detectable NNRTI resistance-associated mutations (RAMs) at baseline. We studied 44 baseline reverse transcriptase (RT) mutations, but only analysed those present in 35 patients.
Results: The following RT mutations were associated with decreased virological response to TMC125 (TMC125 RAMs): V90I, A98G, L100I, K101E/P, V106I, V179D/F, Y181C/I/V and G190A/S. Isolates with A98G, K101E, V179F, Y181C or G190A had more NNRTI RAMs than isolates without these mutations. V179F was never present without Y181C. V179F, Y181V and G190S had the largest impact on response, but were present in <5% of patients.
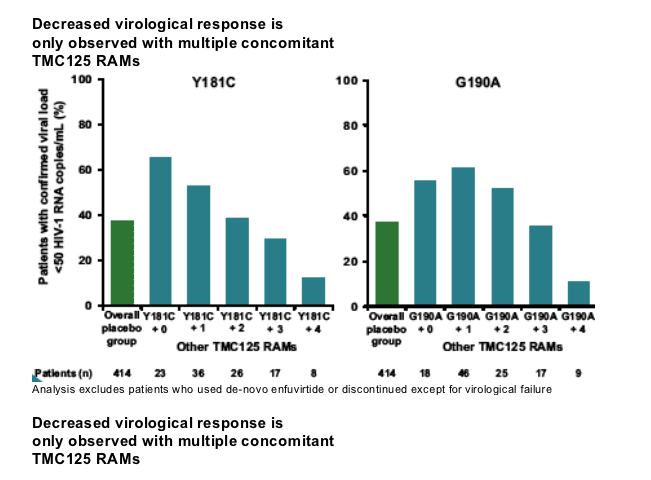
Virological response decreased with increasing numbers of TMC125 RAMs. The largest decrease was in the subgroup of patients with 33 TMC125 RAMs at baseline. The virological response in patients who had Y181C without other TMC125 RAMs (but with other NNRTI RAMs) was comparable to that in patients without detectable NNRTI mutations; a decreased virological response was observed only in the subgroup of patients with Y181C plus two or more TMC125 RAMs.
Conclusions: Thirteen mutations, mainly occurring with other NNRTI RAMs, were associated with a decreased response to TMC125. The decrease was a function of the number of baseline TMC125 RAMs, with the largest impact in the subgroup of patients with 3 or more TMC125 RAMs.
Objectives
To identify the baseline genotypic determinants of decreased virological response to TMC125, in the Phase III DUET-1 and DUET-2 trials (pooled data)
To provide guidance in the interpretation of genotypic resistance information related to TMC125
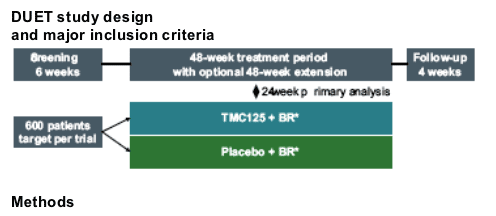
Effect of baseline genotype on the virological response to TMC125 was studied in the subgroup of patients not using enfuvirtide as a new drug, and excluding patients who discontinued for other reasons than virological failure (n=406)
Virological responses in this Week 24 analysis are presented as the proportion of patients who achieved confirmed viral load <50 HIV-1 RNA copies/mL
Decreased virological response was defined as a response that was at least 25% lower than that of the subgroup of patients without detectable NNRTI RAMs at baseline (n=52). These patients had NNRTI mutations from previous genotype
RT mutations were included in the analysis if they were present at baseline in five or more patients
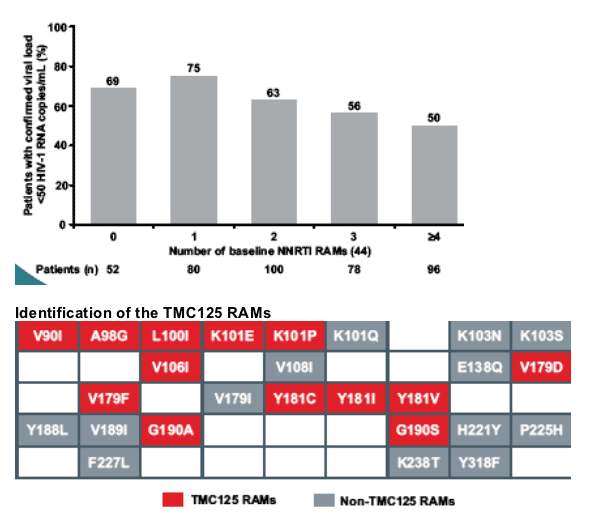
--G190A, K101E, V106I, G190S frequently occurred together with Y181C
--V179F always occurred together with Y181C
--K103N (which is not a TMC125 RAM) did not frequently occur together
with Y181C
The number of baseline TMC125 RAMs
correlated with the virological response
(<50 copies/mL) to TMC125
75% of patients receiving TMC125 who had 0 TMC125 RAMs at baseline achieved <50 c/ml; 60% of patients with 1 TMC125 mutation achieved <50; 58% with 2 TMC125 RAMs achived <50 c/ml; 41% with 3 TMC125 RAMs achieved <50 c/ml; and 25% with 4 or more TMC125 RAMs achieved <50 c/ml.
Analysis excludes patients who used de-novo enfuvirtide or discontinued except for virological failure
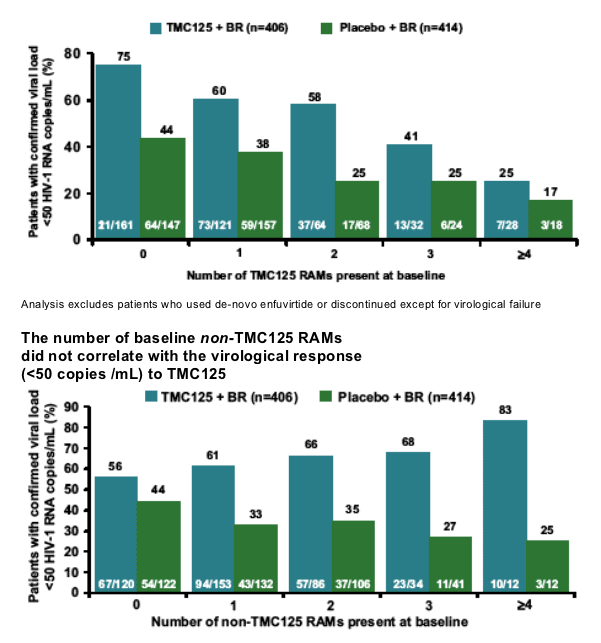
The non-TMC125 RAMs (n=13) are the NNRTI RAMs that were not
identified to have an impact on response
--Analysis excludes patients who used de-novo enfuvirtide or discontinued except for virological failure
|
| |
|
 |
 |
|
|