 |
 |
 |
| |
Prognostic Factors of Virological Success in Antiretroviral-naive Patients Receiving LPV Monotherapy in the MONARK trial
|
| |
| |
Reported by Jules Levin
11th EACS, Oct 24-27, 2007, Madrid
Flandre P, Delaugerre C, Ghosn J, Chaix ML, Horban A, Girard PM, Gladysz A, Cohen Codar I, Ngo Van P, Taburet AM, Rouzioux C, Delfraissy JF
AUTHOR CONCLUSIONS
Naive patients under LPV/r monotherapy (N=66)
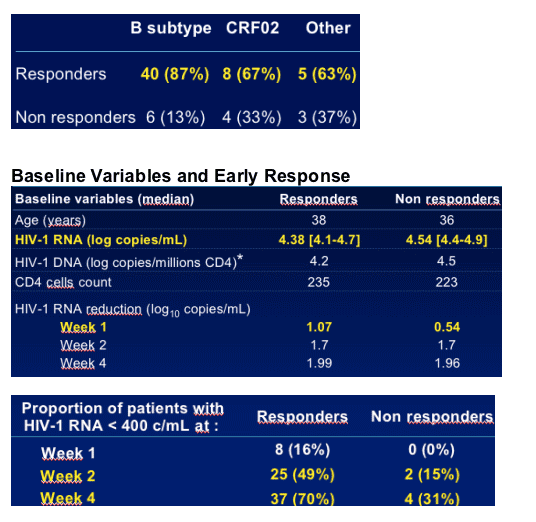
Early virologic response (<400 copies/mL at W4) and HIV-1 subtype (B vs. non B) were independently predictive to response in these patients
ITT analysis showed similar results (baseline HIV-1 RNA and HIV subtype)
Identification of predictive factors to a LPV monotherapy regimen may help to determine which are most likely to benefit from such a strategy
Author discussion
Effect of HIV-1 subtype?
A similar or better early virologic response (Week 4 and Week 16) was observed in non-B subtypes and poorer response thereafter
Multiple potential confounding factors need to be considered:
--Compliance to the regimen
--Difference in demographic variables
True effect ?
MONARK Study Design and Primary Endpoint
Patients received either LPV/r sgc 400/100mg bid, n=83, or LPV/r sgc 400/100 plus AZT/3TC 300/150mg bid, n=53.
Main Entry criteria
- Antiretroviral naive
- HIV-1 RNA <100,000 c/mL
CD4 > 100 cells/mm3
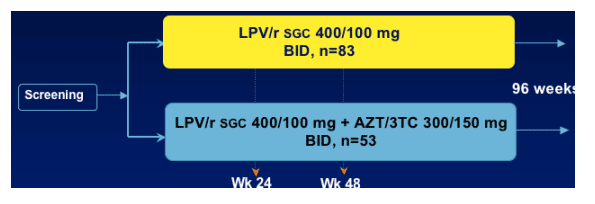
Primary Endpoint
Proportion of subjects with HIV-1 RNA < 400 copies/mL at Week 24
AND < 50 copies/mL at Week 48 Virologic Response
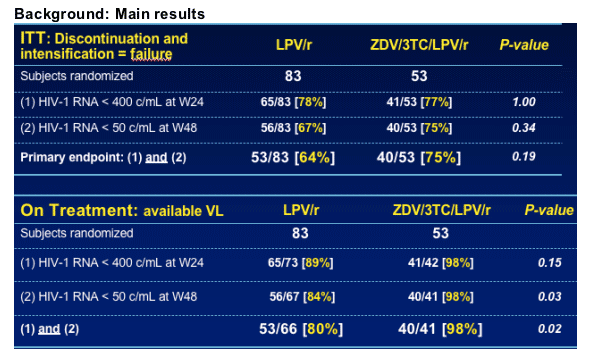
Background: Main results
N=83 patients in LPV/r arm
--13 discontinuations
--3 patients intensified with AZT/3TC
--1 patient with missing VL at week 24
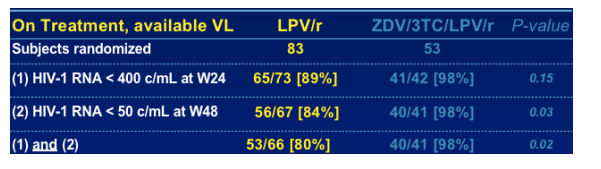
N=66 patients included in the present OT analysis
--53 (80%) responders
--13 (20%) non-responders
OBJECTIVES & METHODS
Objective
To determine predictive factors of virologic response in patients randomized in the LPV/r monotherapy arm
Methods
-- 'On treatment' analysis on patients with available VL
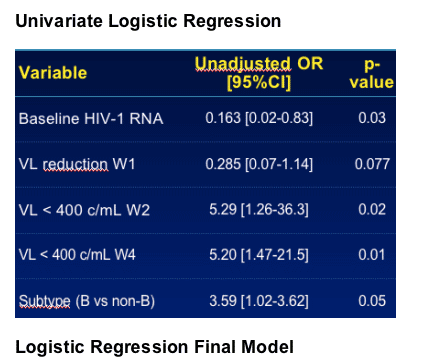
-- Logistic regression model (univariate and multivariate)
VARIABLES INVESTIGATED
Demographic variables
--Gender and Age
Baseline characteristics
--HIV-1 RNA (log10 copies/mL)
--CD4 cell count
--HIV-1 DNA (log10 copies/mL)
HIV-1 subtype
Early virologic response
--HIV-1 RNA decrease at W1, W2 and W4
--HIV-1 RNA levels at W1, W2 and W4 (<400 or <50 copies/mL)
LPV trough concentration at W4, W24 and W48
Compliance evaluation (W4, W8, W12, W16, W20, W24, W32, W40 and W48)
Results
Baseline Characteristics
Gender

HIV-1 subtype
-- B subtype n=46 (70%)
-- Non-B subtypes n=20 (30%)
A n=1
CRF01 n=1
CRF02 n=12 (18%)
CRF03 n=1
CRF06 n=2
F n=1
G n=2
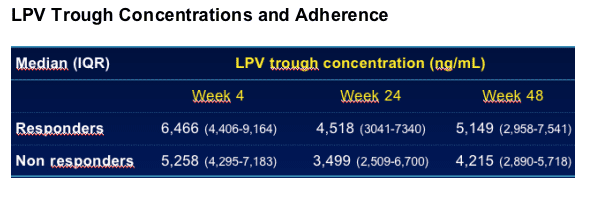
Patients had at least one undetectable (<75 ng/mL) LPV concentration
--Responders 2/53 (4%) vs. Non responders 1/13 (8%)
Patients who declared having missed one dose or more in at least 2 evaluations of compliance
--Responders 10/53 (19%) vs. Non responders 4/13 (31%)
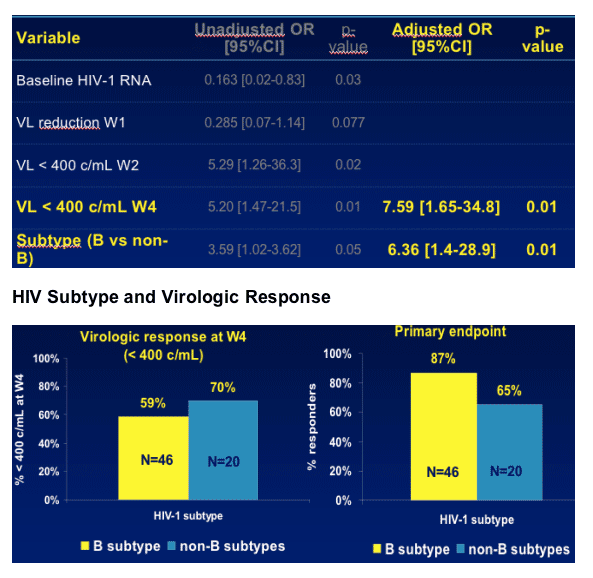
Differences Between B and Non B Subtypes
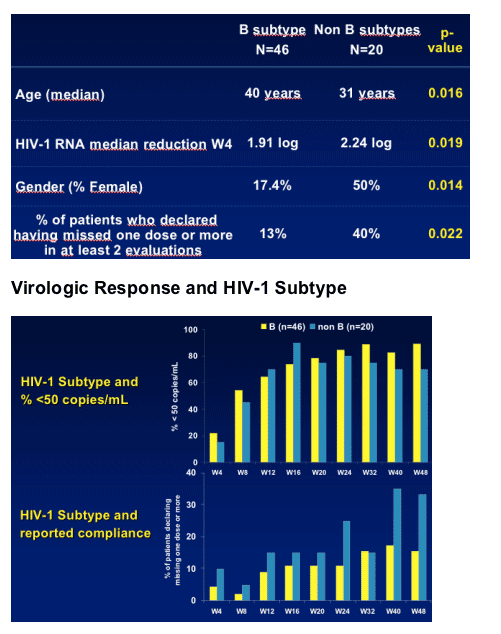
--Compliance : % of patients who declared having missed 1 dose or more in at least 2 evaluations- 13% for B subtype (n=46), 40% for Non B subtypes (n=20). (P=0.022)
--Gender : 17.4% female are B subtype, 50% female are Non B subtype. (p=0.014)
--age. Median age is 40 yrs for B subtype, and 31 years for Non B subtypes (p=0.016)
--HIV-1 RNA. B subtype, week 4= 1.91 log ; Non B subtype week 4= 2.24 log (p=0.019)
|
| |
|
 |
 |
|
|