 |
 |
 |
| |
Single Agent Therapy (SAT) with Lopinavir/ritonavir (LPV/r) Controls HIV-1
Viral Replication in the Female Genital Tract
|
| |
| |
Reported by Jules Levin
11th EACS, Oct 24-27, 2007 Madrid Spain
Yeh RF1,2, Hammill HA3, Fiscus SA4, Rezk NL4, Miguel B1, Kashuba ADM4,
Mayberry C5, Nemecek J1, Lipman BA6, Norton M6, Fath M6, Gathe Jr. JC1
1Therapeutic Concepts, Houston, TX; 2University of Houston, Houston, TX; 3Houston, TX;
4University of North Carolina, Chapel Hill, NC; 5Donald R. Watkins Foundation, Houston, TX;
6Abbott Laboratories, Abbott Park, IL
AUTHOR DISCUSSION
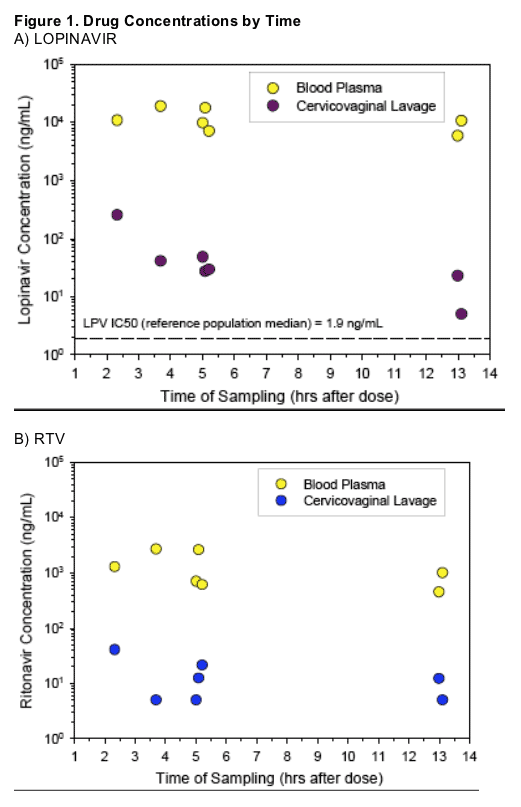
LPV/r single-agent therapy was associated with undetectable genital tract HIV RNA in all 7 studied women who had undetectable HIV in plasma, despite low LPV concentrations in CVL.
LPV concentrations were much lower in CVL samples compared to plasma (median, 0.39% of plasma). All LPV concentrations in CVL except one, exceeded the median reference IC50 for wildtype HIV-1 (1.9 ng/mL) as well as the 99th percentile LPV IC50 for wildtype HIV-1 (5.1 ng/mL).12
Direct aspirate (n=1) LPV concentration was 20.1-fold higher than matched CVL, suggesting CVL concentrations are diluted at least 10-to-20-fold compared to direct aspiration. As sampling was done at a single timepoint, this likely further decreased the measured CVL concentration.
One study determined that concentrations of drug binding proteins in cervical mucus were <1% of blood plasma.13 Thus, free drug concentrations in FGT may
be similar to, or greater than, plasma despite lower total drug concentrations.
Limitations: no baseline pelvic exam for comparison, measurement of total drug concentrations (not free or unbound), and further dilution of cervicovaginal
lavage due to prior direct aspiration limits ability to make direct comparison.
AUTHOR CONCLUSIONS
Control of HIV replication in blood plasma with LPV/r as single-agent therapy is associated with suppression of HIV replication in sanctuary sites of the central nervous system and the male genital tract, but data in the FGT was previously unknown.
This study is the first to show suppression of HIV replication in the FGT in a diverse cohort of na´ve subjects treated with LPV/r single-agent therapy for at
least 48 weeks.
HIV RNA was undetectable (<400 copies/mL) in the cervicovaginal fluid of all 7 women studied.
LPV/r penetration into cervicovaginal fluid exceeded the reference population median IC50 (1.9 ng/mL) in all but one sample, despite significant dilution of lavage samples.
Larger longitudinal investigations are warranted evaluating single-agent therapy on viral suppression and drug pharmacokinetics in sanctuary sites.
INTRODUCTION
Lopinavir/ritonavir (LPV/r) as single agent therapy has demonstrated virologic suppression when used in a variety of treatment strategies.1-5
We have previously shown that LPV/r single-agent therapy suppressed viral replication in the cerebrospinal fluid of 10 out of 11 antiretroviral-na´ve subjects.6
A separate study (MONARK) evaluated viral response in semen and showed undetectable HIV RNA in 5 out of 5 men on LPV/r single-agent therapy after 48 weeks of therapy, despite undetectable semen LPV and ritonavir (RTV) concentrations.7
Use of combination antiretroviral therapy has been associated with decreased genital HIV shedding in men and women.8,9
When used as part of combination therapy, LPV concentrations in cervicovaginal fluid (CVF) from direct aspiration were a median of 8% (interquartile range, 0.4%, 245%) of blood plasma.10
No study to date has evaluated control of HIV replication and LPV/r penetration into the female genital tract (FGT) with LPV/r single-agent therapy.
OBJECTIVES
To assess viral load in the FGT after ≥ 24 weeks of LPV/r single-agent therapy in patients that have HIV RNA <75 copies/mL on at least 2 consecutive visits.
To estimate LPV/r penetration into the FGT compared to blood plasma
METHODS
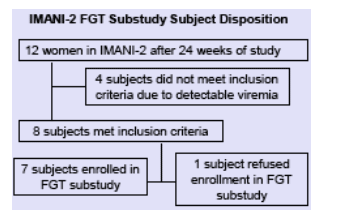
IMANI-2 is an ongoing, prospective, open-label investigation of LPV/r single-agent therapy in 39 antiretroviral-na´ve, HIV-1-infected subjects administered as 400/100mg twice daily.
This substudy was approved by the Institutional Review Board and all subjects provided written, informed consent.
Inclusion criteria: All females on at least 24 weeks of LPV/r with at least 2 consecutive plasma HIV RNA <75 copies/mL by branched DNA assay at least 4
weeks apart and at the most recent evaluation were approached for enrollment
Exclusion criteria: not meeting inclusion criteria
Paired blood plasma, FGT sampling
Genital tract sampling was performed within 14 days after end of menses in menstruating women. Prior to FGT sampling, women refrained from sexual
intercourse, vaginal medications, and douching for at least 72 hours.
Pelvic exams were performed by a board-certified obstetrician-gynecologist
Adherence over the previous week was based on 7-day, 3-day, and 24-hour self-report. Subjects documented the exact time of the last dose prior to FGT sampling.
Order of FGT sampling: 1) direct CVF aspirate using vaginal aspirator (for LPV, RTV concentrations), 2) direct cervical wicking from cervix using Tearflo
strips for HIV RNA quantitation, 3) direct wicking from vagina using Tearflo strips for HIV RNA, and 4) cervicovaginal lavage (CVL) for LPV, RTV concentrations
Cervicovaginal lavage: 1mL nonbacteriostatic, sterile sodium chloride (0.9%) directed at endocervical os and removed using vaginal aspirator
ASSAYS
HIV RNA: Plasma HIV RNA by branched DNA assay (Bayer Versant HIV RNA 3.0), lower limit of quantitation (LLOQ) = 75 copies/mL; cervical, vaginal wicks by RT-PCR (NucliSens, bioMerieux), LLOQ = 400 copies/mL
LPV, RTV drug concentrations11: HPLC/UV, LLOQ= 25 ng/mL in plasma; LC/MS/MS, LLOQ = 10 ng/mL in CVF, CVL
ANALYSES/ STATISTICS
For the purpose of calculating CVL:Plasma ratios, CVL concentrations below the limit of quantitation (BLQ) were set at 1/2 of the LLOQ.
Data are presented as median [range] unless otherwise indicated.
RESULTS
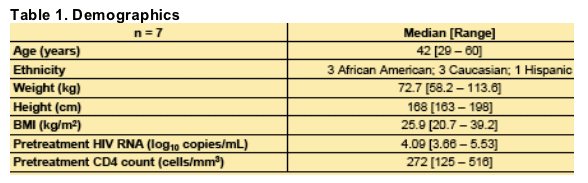
In all women, the main risk factor for HIV transmission was heterosexual intercourse. Median number of sexual partners in the past 6 months was 1 [0 - 2]. Median lifetime sexual partners = 5 [1 - >10].
4 women were no longer menstruating: 3 with history of total abdominal hysterectomy, 1 with total vaginal hysterectomy.
Pelvic exams and CVF sampling were performed at median of 81 [54 - 88] weeks after LPV/r initiation.
Viral Suppression
All subjects achieved plasma HIV RNA suppression to <75 copies/mL by week 16 after LPV/r initiation. Median time of suppressed plasma HIV RNA at time of FGT sampling was 69 [50 - 84] weeks.
All 7 subjects had vaginal HIV RNA <400 copies/mL, and all 3 subjects with an intact cervix had cervical HIV RNA <400 copies/mL, at the time of sampling.
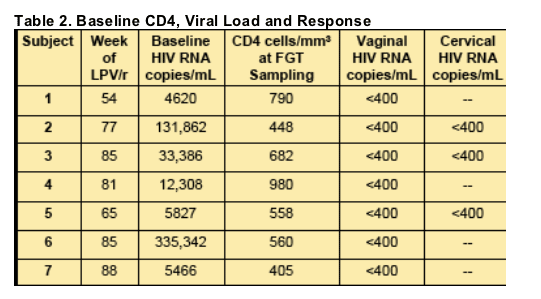
Drug Penetration
Direct cervicovaginal aspirate was able to be done in only 1 woman.
Cervicovaginal lavage (CVL) performed in all.
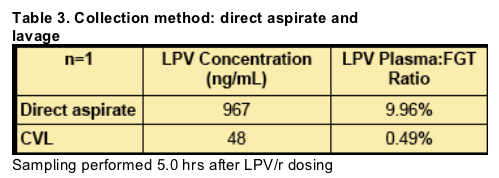
CVL
_ One sample had LPV concentrations that were
detectable but BLQ.
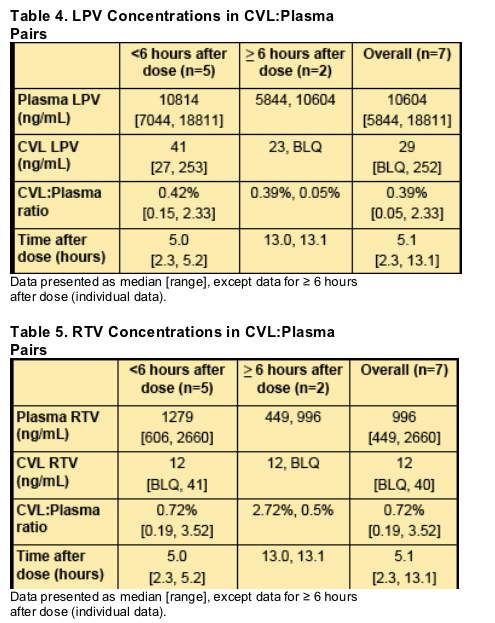
_ Three samples had detectable RTV, but were BLQ.
References
1. Gathe Jr. JC, Yeh RF, Mayberry C, et al. Single-agent Therapy with Lopinavir/ritonavir Suppresses Plasma HIV-1 Viral Replication at 24 and 48 Weeks. XVII International AIDS Conference; Sydney, Australia. July 22-25,
2007. Abstract 1518.
2. Defrais JF, Flandre P, Delaugerre C, et al. MONARK Trial: 48-week analysis of lopinavir/ritonavir monotherapy compared to LPV/r + zidovudine/lamivudine in antiretroviral-naive patients. XVI International AIDS Conference; Toronto,
Canada. August 13-18, 2006. Abstract THLB0202.
3. Cameron W, da Silva B, Arribas J, et al. A two-year randomized controlled clinical trial in antiretroviral-naive subjects using lopinavir/ritonavir monotherapy after initial induction treatment compared to an efavirenz 3-drug regimen (Study M03-613). XVI International AIDS Conference; Toronto, Canada. August 13-18, 2006. Abstract THLB0201.
4. Nunes EP, Oliveira MS, Almeida MMTB, et al. 48-week efficacy and safety results of simplification to single agent lopinavir/ritonavir regimen in patients suppressed below 80 copies/mL on HAART - the KalMo study. XVI International AIDS Conference; Toronto, Canada. August 13-18, 2006. Abstract TUAB0103.
5. Arribas J, Pulido F, Delgado R, et al. Lopinavir/ritonavir as single-drug maintenance therapy in patients with HIV-1 viral suppression: forty eight week results of a randomized, controlled, open label, clinical trial (OK04 Study). XVI International AIDS Conference; Toronto, Canada. August 13-18, 2006. Abstract THLB0203.
6. Yeh RF, Letendre S, Novak I et al. Single-agent therapy with lopinavir/ritonavir controls HIV-1 viral replication in the central nervous system. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, California. February 25-28, 2007. Abstract E-177.
7. Ghosn J, Peytavin G, Galimand J, et al. Absence of viral shedding in the male genital tract after one year of lopinavir/ritonavir alone or in combination with Combivir«:a substudy of Monark trial. 15th International Drug Resistance
Workshop; Sitges, Spain. June 13-17, 2006. Abstract 76.
8. Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatemnt of HIV-infection results in suppression of the seminal shedding of HIV. AIDS
2000;14:117-121.
9. Cu-Uvin S, Caliendo AM, Reinert S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS 2000;14:415-421.
10. Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 2007;21:1899-1907.
11. Rezk NL, Crutchley RD, Yeh RF, Kashuba ADM. Full Validation of an Analytical Method For The HIV Protease Inhibitor Atazanavir In Combination With 8 Other Antiretroviral Agents and Its Applicability to Therapeutic Drug Monitoring. Ther Drug Monit 2006;28:517-525.
12. Parkin NT, Hellmann NS, Whitcomb JN, et al. Natural variation in drug susceptibility in wild type human immunodeficiency virus-1. Antimicro Agents and Chemother 2004;42(2):437-443.
13. Salas HIG, Pearson RM, Turner P. Quantitation of albumin and alpha-1-acid glycoprotein in human cervical mucus. Hum Exp Toxicol 1991;10:137-139.
|
| |
|
 |
 |
|
|