 |
 |
 |
| |
Single agent lopinavir/ritonavir (LPV/r) is as effective at maintaining viral suppression as continuation of standard ART at 24 weeks in patients who already have HIV RNA <50 copies/mL
|
| |
| |
Reported by Jules Levin
11th EACS, Oct 24-27, 2007, Madrid Spain
Kasha Singh1, Alastair Teague1, Akil Jackson1, Sifiso Mguni1, Chris Higgs1, Sundhiya Mandalia2, Brian Gazzard1, Mark Nelson1
1Chelsea and Westminster Hospital, London, UK; 2 Imperial College, London, UK
INTRODUCTION
Use of combination antiretroviral therapy is efficacious in controlling HIV replication but is constrained by toxicity and cost. Monotherapy may reduce these and involve fewer tablets with less potential for drug interactions and adverse events.
OBJECTIVES
We assessed the feasibility of reducing treatment to single agent LPV/ r maintenance therapy in HIV patients on standard antiretroviral therapy (ART) with viral load (VL) <50 copies/mL. Patients were eligible with up to four protease inhibitor (PI) mutations1. Results at week 24 are presented.
METHODS
Patients with HIV-1 infection who had been taking ART (≥ three agents) with HIV
VL <50 copies/mL for at least six months and CD4 >200 cells/mL were recruited to this single centre, open label, 48-week study. Up to four PI mutations were allowed. Subjects were randomised to continue ART or change to LPV/r
monotherapy (400/100 mg BID). HIV VL, CD4, haematology and biochemistry
were tested at baseline and at weeks 4, 12, 24, 36 and 48. Rates of maintenance of HIV VL <50 copies/mL and change in CD4 cell count were calculated with
planned interim analysis at 24 weeks. Results were analysed by intent-to-treat, with missing values equalling failure. Estimates of change in CD4 cell count and cholesterol were obtained from intervention by time interaction.
RESULTS
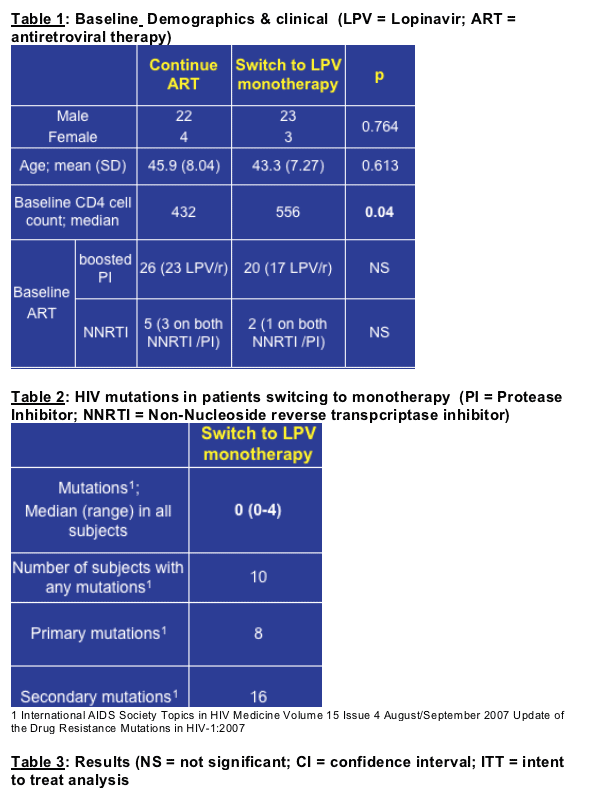
52 subjects were enrolled of whom 45 were male. 26 (23 male) were randomised
to switch to LPV/r monotherapy. Mean baseline CD4 cell count was higher in the
monotherapy arm (556 cells/mL vs. 432 cells/mL, p = 0.04). Of those subjects
continuing ART, 26 were taking a boosted PI; in 23 of these subjects the boosted
PI was LPV/r. Five subjects were taking a non-nucleoside reverse transcriptase
inhibitor as part of their ART combination. In the monotherapy arm the median
(range) of PI resistance mutations documented was 0 (0-4) per subject. Ten
subjects had a total of 24 mutations altogether (median 2, range 1-4). Eight of the
24 mutations were primary for LPV/r1 (Table 2).
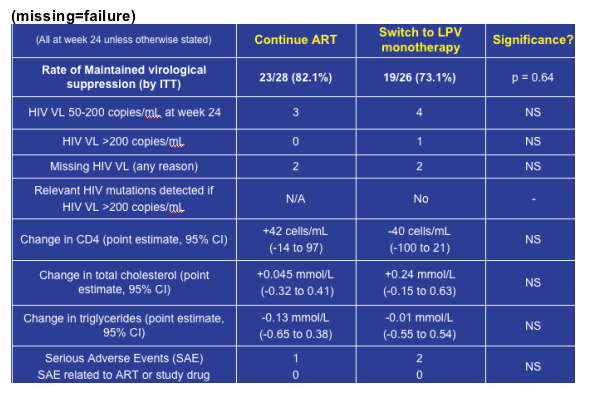
At week 24 no significant difference was seen in the rate of maintained virological
suppression for subjects continuing ART (23/28 (82.1%)) compared with (19/26
(73.1%)) of those on monotherapy (p=0.64). In seven subjects HIV VL was 50-200 copies/mL (3 ART, 4 monotherapy). In one subject on the monotherapy arm HIV VL was >100,000 copies/mL at week 24. In this subject VL was <50 copies/mL at weeks 4 and 12. LPV/r resistance mutations had not been detected in this subject at any time in the past and genotype at week 24 showed wild type virus (Table 3).
Results were missing for three subjects who failed to attend the week 24 visit (2
ART, 1 monotherapy). One subject withdrew from the study at week 12 and this
was due to monotherapy intolerance (diarrhoea, Grade 2 severity (DAIDS
grading)). A total of three serious adverse events (1 ART, 2 monotherapy) were
reported in three subjects. None of these was believed to be related to ART or
study drug (Table 3) and all resolved within seven to ten days.
Change in CD4 at week 24 (point estimate, 95% confidence intervals) in the standard ART arm was +42 cells/mL (-14 to 97) and -40 cells/mL (-100 to 21) in the monotherapy arm (Table 3). Although a small significant increase in total cholesterol was observed at 4 and 12 weeks in the LPV/r monotherapy group, there was no significant change in total cholesterol/triglycerides in either group at 24 weeks (Table 3).
CONCLUSIONS
There was no significant difference between LPV/r monotherapy and continuing standard ART in the rate of maintenance of viral suppression at 24 weeks in subjects with up to 4 PI mutations.
1 International AIDS Soc Topics in HIV Med Vol 15 Iss 4 Aug/Sept 2007 Update of Drug Resistance Mutations in HIV-1:2007
|
| |
|
 |
 |
|
|