 |
 |
 |
| |
Once-Daily Fosamprenavir (FPV) Boosted with Either 100mg or 200mg of
Ritonavir (r) Along with Abacavir (ABC)/Lamivudine (3TC): 48 Week Safety and Efficacy Results from COL100758
|
| |
| |
Reported by Jules Levin
11th EACS, Oct 24-27, 2007 Madrid Spain
C Hicks1, E DeJesus2, D Wohl3, Q Liao4, K Pappa4, T Lancaster4
1Duke Univ. Med. Ctr., Infectious Diseases, Durham, USA, 2Orlando Immunology Ctr., Orlando, USA,
3University of North Carolina at Chapel Hill, Chapel Hill, USA, 4GlaxoSmithKline, Research Triangle Park, USA
On Oct 12, 2007-" The U.S. Food and Drug Administration approved once-daily LEXIVA 1400 mg with 100mg of ritonavir in adult patients who had not previously taken a protease inhibitor (PI). The approval was based on pharmacokinetic data demonstrating comparable blood plasma levels in healthy volunteers when LEXIVA was administered with the lower 100mg dose of ritonavir and the previously approved 200mg dose of ritonavir. This information has been added to the LEXIVA product label".
AUTHOR DISCUSSION
FPV/r100-treated patients generally had better virologic responses and similar CD4+ T-cell count improvements compared to FPV/r200-treated patients.
The higher virologic efficacy rate but absence of tolerability or lipid advantages observed with FPV/r100 in this study may in part be related to the greater adherence FPV/r100-treated patients had to RTV and ABC/3TC, as well as to differences in baseline characteristics compared to FPV/r200-treated patients (fewer CDC class C patients, lower overall baseline viral load, and higher CD4 cell counts).
There was a higher study discontinuation rate in the FPV/r200 arm, but given the small sample size, it's difficult to determine whether this was related to the regimen.
AUTHOR CONCLUSION
In this study, efficacy through 48 weeks favored the FPV/r 1400mg/100mg arm, perhaps due to baseline characteristics and adherence. Both arms showed similar improvements in CD4 cell count and Total/HDL cholesterol ratio.
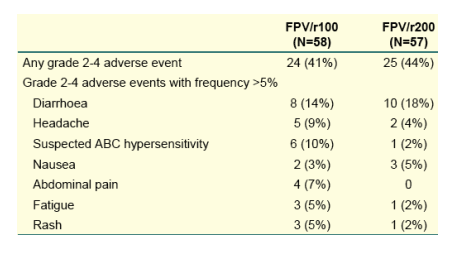
Better regimen adherence was observed in patients taking 100 mg ritonavir as compared with 200 mg ritonavir.
Abstract
Background: The once-daily (QD) dose of FPV/r most studied in clinical trials has been 1400mg/200mg. Although pharmacokinetic data in healthy volunteers supports QD dosing with FPV/r 1400mg/100mg, clinical data in HIV-infected patients (pts) supporting the FPV/r 1400mg/100mg QD dose is limited.
Methods: This open-label, randomized study compared efficacy and safety of FPV/r 1400mg/100mg (FPV/r100) to FPV/r 1400mg/200mg (FPV/r200) + ABC/3TC 600mg/300mg given QD in 115 ART-naive pts. Summary results and p-values were descriptive only. Adherence was measured using pill counts. Prospective HLA-B*5701 allele testing was not done.
Results: FPV/r100 vs FPV/r200 baseline (BL) data were: median HIV RNA
viral load (VL) 4.7 vs 4.9 log10c/mL, median CD4 259 vs 179 cells/mm3,
Total/HDL-cholesterol ratio 4.6 vs 4.6, males 81% vs 81%, non-white 63%
vs 55%.
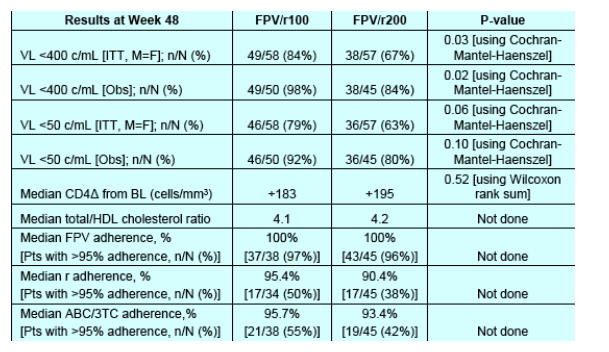
--Viral load <50 c/ml [ITT, M=F] is 79% [46/58] for FPV/r100mg and 63% for FPV/r200mg [p=0.06].
--Viral load <400 c/ml [ITT, M=F] IS 84% [49/58] for FPV/r100mg and 67% for FPV/r200mg [==0.03].
--median cd4 delta increase from baseline was 183 for FPV/r100 and 195 for FPV/r200.
--adherence appears to better with the FPV/r100 than the FPV/r200 regimen.
Treatment-related grade 2-4 adverse events (AEs) ≥5% were diarrhea 14%
vs 18%, headache 9% vs 4%, suspected ABC HSR 10% vs 2%, abdominal
pain 7% vs 0%, nausea 3% vs 5%, fatigue 5% vs 2%, and rash 5% vs 2%.
Conclusions: In this study, efficacy through 48 wks favored FPV/r 1400mg/100mg, perhaps due to baseline characteristics and adherence. Both arms showed similar improvements in CD4 cell count and Total/HDLcholesterol ratio.
INTRODUCTION
Until recently, the ritonavir dose approved for providing pharmacologic boosting of a once-daily fosamprenavir regimen was 200 mg.
Pharmacokinetic analyses support the use of 100 mg ritonavir to provide therapeutic concentrations of plasma amprenavir1, and 100-mg dosing has now been approved in the US on this basis.
To date, there have not been any prospective studies that compare long-term safety and efficacy of 100 mg versus 200 mg ritonavir-boosting of once-daily fosamprenavir regimens. Thus, a 96-week study was initiated to evaluate efficacy and safety of the 100 mg dose in comparison to 200 mg of ritonavir.
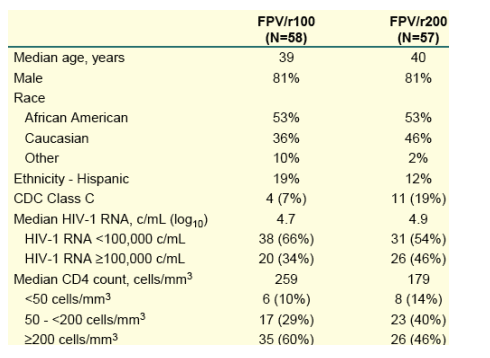
The study enrolled 115 subjects. Baseline characteristics are shown in Table 1, and have previously been described2.
Table 1. Baseline Characteristics
66% in the FPV/r100 arm and 54% in the FPV/r200 arm had <100,000 c/ml at baseline. 34% in the FPV/r100 arm and 46% in the FPV/r200 arm had >100,000 c/ml. median cd4 count was 259 in the FPV/r arm and 179 in the FPV/r200 arm. 10% in the FPV/100 arm and 14% in the FPV/200 arm had <50 cd4s. 40% in the FPV/r200 arm and 29% in the FPV/r100 arm had cd4 count 50-200. 60% in the FPV/r100 arm and 46% in the FPV/r200 arm had >200 cd4s. 53% of study participants are African-American. 19% in the FPVr/r100 arm and 12% in the FPV/r200 arm are Hispanic.
METHODS
COL100758 is a 96-week, open-label, multicenter study that enrolled adult antiretroviral-naive, HIV-infected patients (HIV-1 RNA >1000 copies/mL), who were randomized to receive once daily abacavir 600 mg/lamivudine 300 mg plus fosamprenavir 1400 mg boosted with 100 mg versus 200 mg of ritonavir.
The objectives were to evaluate efficacy, immunological and metabolic responses, safety, and tolerability of the 100mg ritonavir versus the 200mg ritonavir regimen, as well as to evaluate adherence, over 24, 48, and 96 weeks. The study was not powered and p-values provided are descriptive only.
The primary efficacy endpoint was the proportion of subjects with plasma HIV-1 RNA <400 copies/mL at Week 48. Results from the intent to treat, missing equal to failure (ITT, M=F) and observed (Obs) analyses for the efficacy endpoints are presented. The comparisons between the two arms were made by Cochran-Mantel-Haenszel test.
Differences in CD4+ T-cell count were compared by Wilcoxon rank sum test.
RESULTS
Table 2. Subject Disposition at End of Week 48
90% in the FPV/r100 arm and 77% in the FPV/r200 arm completed 48 weeks; 10% taking FPV/r100 and 23% taking FPV/r200 prematurely withdrew. Primary reasons for withdrawal: adverse event was the primary reason for withdrawal for 2% taking FPV/r100 and for 4% taking FPV/r200; protocol defined virologic failure: 0 in FPV/r100 and 2% in FPV/r200; non-compliance: 2% FPV/r100, 4% FPV/r200; lost to follow-up: 5% FPV/r100, 11% FPV/r200.
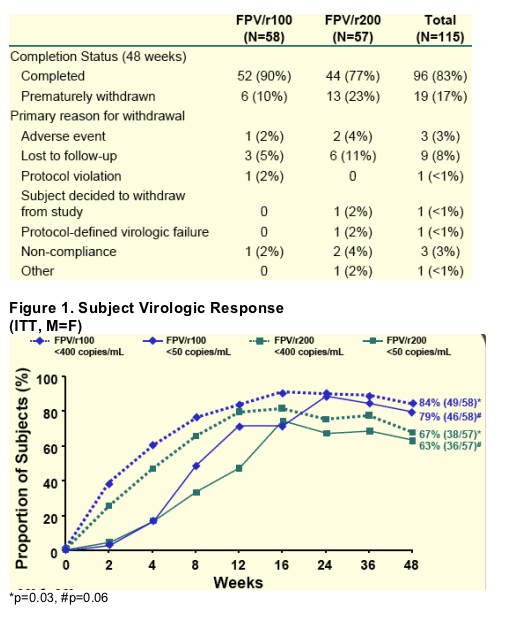
In the observed analysis, the proportions of subjects with HIV RNA <400 c/mL were 98% vs 84% (p=0.02), for FPV/r100 vs FPV/r200; and the proportions were 92% vs 80% (p=0.10), respectively, for <50 c/mL.
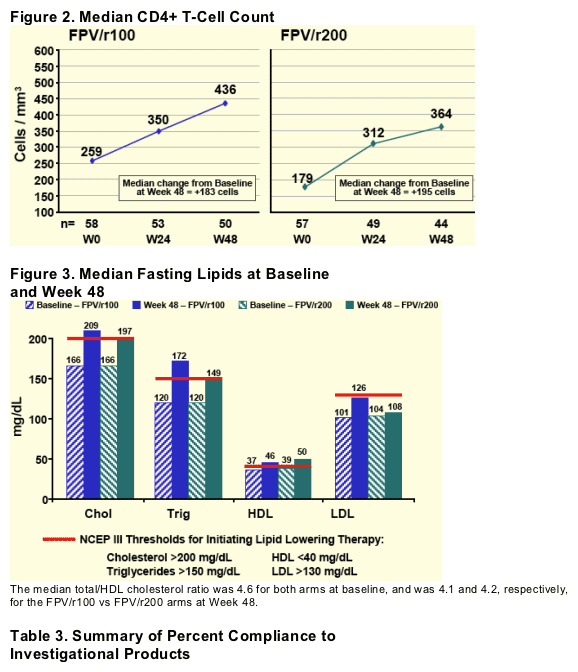
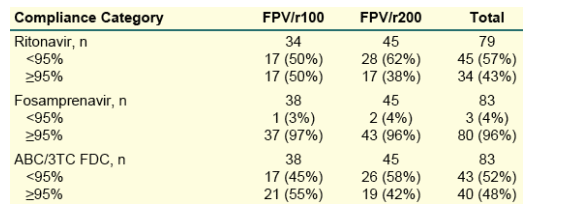
Table 4. Summary of Treatment-Related
Adverse Events
Any grade 2-4 adverse event: 41% FPV/r100; 44% FPV/r200.
Grade 2-4 adverse events with >5% frequency:
Diarrhea: 14% FPV/r100, 18% FPV/r200
Headache: 9% FPV/r100, 4% FPV/r200.
Suspected ABC hypersensitivity: 10% FPV/r100, 2% FPV/r200
Nausea: 3% FPV/r100, 5% FPV/r200
Fatigue: 5% FPV/r100, 2% FPV/r200
Rash: 5% FPV/r100, 2% FPV/r200
|
| |
|
 |
 |
|
|