 |
 |
 |
| |
Patients who responded to tipranavir/r (500/200 mg BID) plus new enfuvirtide (ENF) at Week 16 of RESIST studies maintain superior virologic and immunologic outcomes through Week 96; effects of adding 1 new class to potent protease inhibitor in highly treatment-experienced patients
|
| |
| |
Reported by Jules Levin
11th EACS, Oct 2007, Madrid, Spain
G Pierone Jr1, B Clotet2, D Jayaweera3, D Neubacher4, P Piliero5
1Treasure Coast Infectious Disease Consultants, Vero Beach, USA; 2Hospital Universitari Trias I Pujol, Barcelona,
Spain; 3University of Miami, Miami, USA; 4Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany;
5Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA
Author Conclusions
RESIST patients taking TPV/r plus 'new' ENF experienced highly effective antiviral activity, which was sustained in most patients throughout 96 weeks
compared to those taking CPI/r and 'new' ENF. Immunologic outcomes were also greater in the TPV/r plus new ENF group.
Over 70% of patients who took new ENF and TPV/r who had VL <400 copies/mL at Week 16 maintained this level of viral suppression through Week 96.
Administering TPV/r plus a novel ARV results in substantial virologic and immunologic responses in a high proportion of treatment experienced patients.
Abstract
Background Current guidelines recommend >2 active ARVs in treatment
experienced patients (pts) to achieve undetectable viral load (VL) and improved
immunologic response.
Aim To evaluate virologic and immunologic efficacy of tipranavir/r (TPV/r) plus a novel class ARV, e.g. enfuvirtide (ENF) in ARV experienced pts. Analysis focused on RESIST pts who took ENF and had early virologic response (VL <400 copies/mL). Virologic and immunologic efficacy was assessed over 96 weeks.
Methods Analysis of Wk 2, 16, 24, 48 and 96 efficacy data from RESIST.
Results 16.6% (124/746) of TPV/r and 97/737 (13.2%) CPI/r pts initiated ENF for first time (new ENF). At Wk 2, mean VL reduction from baseline in new ENF pts who took TPV/r was 1.62 vs. 1.11 log10 copies/mL in those who took TPV/r without ENF. At Wk 16, 66/124 (53.2%) of new ENF pts in TPV/r arm had VL <400 copies/mL vs. 29/97 (29.9%) in CPI/r arm (ITT NCF).
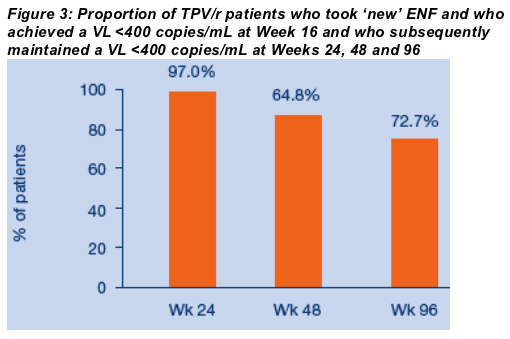
All but two of TPV/r pts (64/66; 97%) maintained VLs <400 copies/mL at Wk 24; 56/66 (84.8%) at Wk 48; and 48/66 (72.7%) at Wk 96 (ITT NCF).
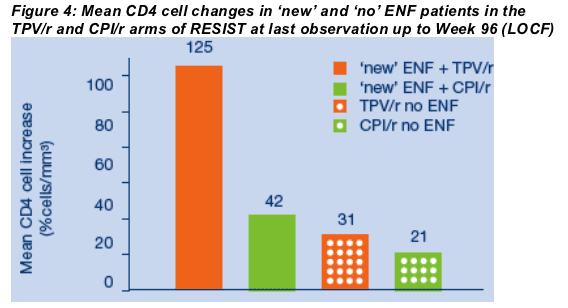
At last observation up to Wk 96, mean increase in CD4 cell count (cells/mm3) in new ENF pts was 125 in TPV/r vs. 42 in CPI/r (LOCF).
Conclusions RESIST patients taking TPV/r plus new ENF had superior early and durable virologic and immunologic outcomes vs. those taking CPI/r and new ENF. The majority of new ENF/TPV/r patients who had VL <400 copies/mL at Week 16 maintained this level of viral suppression through Week 96. Administering tipranavir/r plus a novel ARV results in substantial virologic and
immunologic responses in a high proportion of treatment experienced patients.
Introduction
· Currently, guidelines recommend the use of at least two active antiretroviral (ARV) drugs in the treatment of HIV positive patients who have failed previous therapeutic regimens [1,2]. The aim is to suppress viral replication and increase the CD4 cell count, thus protecting the patient from opportunistic infections and disease progression.
· The DHHS guidelines state: Adding a drug with activity against drug-resistant virus (e.g., a potent ritonavir-boosted PI) and a drug with a new
mechanism of action (e.g., HIV entry inhibitor) to an optimized background
antiretroviral regimen can provide significant antiretroviral activity [1].
In addition: Tipranavir and darunavir are two new protease inhibitors approved for patients who are highly treatment experienced or have HIV-1 strains resistant to multiple PIs based on its demonstrated activity against
PI-resistant viruses [1].
· The BHIVA guidelines acknowledge that 'The goals of treatment for the majority of treatment experienced patients have changed, and the new paradigm should be aiming for an undetectable and durable HIV plasma viral load suppression, wherever possible, leading to immunological improvement with lack of clinical progression and improvement in quality of life' [3].
· In the RESIST studies, patients who had prior experience with all three ARV drug classes including more than one protease inhibitor (PI) switched to tipranavir/r (TPV/r), enfuvirtide (ENF) and an optimized background regimen [4].
· The aim of this analysis was to evaluate the virologic and immunologic efficacy of TPV/r with or without ENF in treatment experienced patients who took part in the RESIST studies. The analysis focused on RESIST patients who took ENF for the first time ('new' ENF) and had an early virologic response (VL <400 copies/mL at Week 16). Virologic and immunologic efficacy was assessed over 96 weeks.
Methods
An analysis of Week 2, 16, 24, 48 and 96 efficacy data from RESIST was conducted, using data from patients in the RESIST studies who took TPV/r or a comparator ritonavir boosted protease inhibitor (CPI/r) with or without 'new' ENF. All virologic analyses were Intent to Treat Non-Completer equals Failure (ITT NCF) unless otherwise stated. The immunologic analysis was Last Observation Carried Forward (LOCF).
Results
Approximately one quarter of TPV/r patients took ENF: 170/746, 22.8%. One hundred and twenty-four patients in the TPV/r arm (16.6%; 124/746) initiated ENF for the first time ('new' ENF), while 6.2% (46/746) recycled or continued to take ENF ('old' ENF) at study initiation. There were 576 TPV/r patients who did not take ENF (576/746; 77.2%). A smaller proportion of CPI/r patients initiated ENF for the first time compared to TPV/r patients: 97/737 (13.2%).
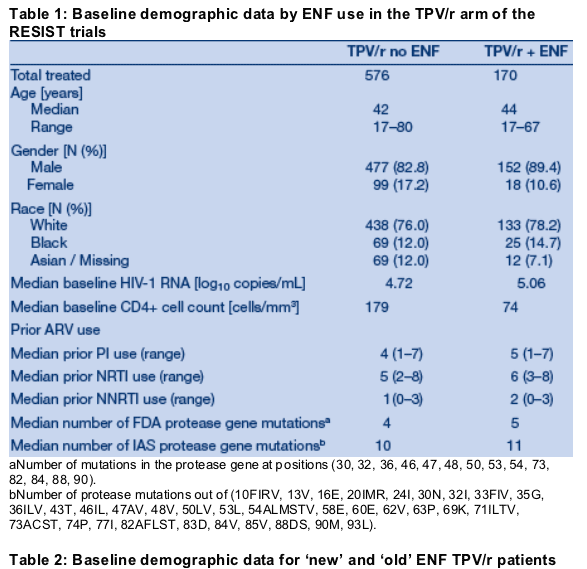
Patients who took TPV/r plus ENF had more advanced HIV disease at baseline
than patients who did not take ENF: median VL: 5.06 vs. 4.72 log10 copies/mL; median CD4 cell count: 74 vs. 179 cells/mm3; proportion of patients with CDC Class C HIV disease: 65.3% vs. 57.8% (Table 1). Patients who took TPV/r plus
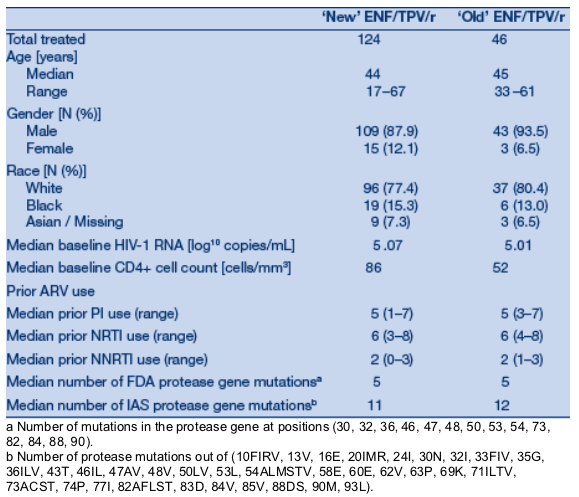
ENF had taken more ARVs prior to enrollment than those who took TPV/r without ENF. The proportion of patients who were hepatitis co-infected was lower in the group of patients who took ENF: 5.3% vs. 11.8%. Other baseline characteristics, however, were similar. There were no differences between the 'new' and 'old' ENF groups (Table 2).
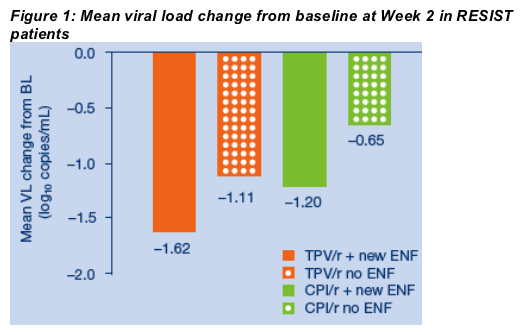
At Week 2, the mean VL reduction from baseline in 'new' ENF patients who took TPV/r was 1.62 vs. 1.11 log10 copies/mL in those who took TPV/r without ENF (Figure 1). The comparable figures for the CPI/r arm were: -1.20 and -0.65 log10 copies/mL in 'new' and 'no' ENF patients, respectively.
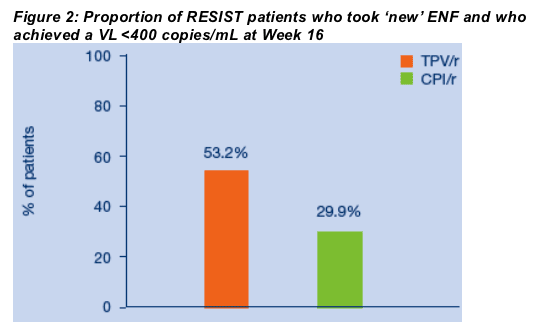
At Week 16, 66/124 (53.2%) of new ENF patients in the TPV/r arm had VL <400 copies/mL vs. 29/97 (29.9%) in CPI/r arm (ITT NCF) (Figure 2). All but two of these 66 TPV/r patients (64/66; 97%) maintained VLs <400 copies/mL at Week 24; 56/66 (84.8%) at Week 48; and 48/66 (72.7%) at Week 96 (ITT NCF) (Figure 3).
At last observation up to Week 96, mean increases in the CD4 cell count in 'new' ENF patients were 125 cells/mm3 in the TPV/r arm versus only 42 cells/mm3 in the CPI/r arm (LOCF) (Figure 4). At last observation up to Week 96, the mean increase in the CD4 cell count in TPV/r patients who did not take ENF was 31 cells/mm3 versus 21 cells/mm3 in CPI/r patients who did not take ENF (LOCF).
References
1. Department of Health and Human Services (DHHS) and Henry J. Kaiser Family Foundation. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. 2006 October: Available from: http://www.hivatis.org.
2. Hammer SM, et al. Jama 2006; 296(7): 827-43
3. Gazzard B, et al. HIV Med 2006; 7(8): 487-503
4. Hicks CB, et al. Lancet 2006; 368(9534): 466-75
|
| |
|
 |
 |
|
|