 |
 |
 |
| |
Tipranavir/r (TPV/r) maintains long term virological suppression
- Three year follow-up of RESIST
|
| |
| |
Reported by Jules Levin
11th European AIDS Conference (EACS),
Madrid, Spain
24-27 October 2007
C Hicks1, P Cahn2, D Ward3, A Lazzarin4, A Jelaska5, M Drulak5, R Vinisko5
on behalf of the RESIST 1 and 2 investigators
1Duke University, Durham, NC, USA; 2Fundación Huesped, Buenos Aires, Argentina;
3Dupont Circle Physicians Group, Washington DC, USA; 4San Raffaele Sci Inst, Milan, Italy;
5Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA
Author Conclusions
⋅ In this highly treatment experienced population, patients who responded to TPV/r experienced durable responses lasting more than 3 years.
⋅ TPV/r treatment response rates were approximately twice those of CPl/r and were four-fold greater for those patients who took enfuvirtide for the first time.
⋅ The PEY-adjusted adverse event profile was similar between the TPV/r and CPl/r groups. Gastro intestinal AEs were less common in the TPV/r arm than the CPI/r arm.
⋅ There were no new safety findings that emerged with TPV/r administration for ≥3 years. In general, emergence of safety events was lower after Week 48, than prior to Week 48.
Abstract
RESIST 1 and 2 are ongoing, randomized Phase III studies of TPV/r (500/200 mg BID) or CPl/r, plus OBR in HTE patients. At Week 156, 218/1483 patients were still taking assigned study medication: 155 TPV/r; 63 CPI/r. Greater treatment discontinuation occurred in CPl/r arm:
TPV/r patient exposure years (PEY) was 2X CPl/r (1315.3 vs. 688.5 PEY).
Discontinuation was predominantly from virologic failure (TPV/r: 201/746 (26.9%); CPl/r: 352/737 (47.8%)).
Previous analyses demonstrated that patients who had added a new class of ARV (ENF) with TPV/r or CPl/r had improved responses. At >3 years, the patients adding new ARV class achieved four-fold greater treatment response rates for TPV/r vs. CPl/r (37.9% vs. 8.2%). Their proportions <50 cp/mL were 21.8% versus 9.3%, respectively.
For the overall study population at >3 year, the percentages of TPV/r patients <50 cp/mL, <400 cp/mL and treatment response were twice that of CPI/r. Median VL decreases from baseline were -0.57 log10 copies/mL in TPV/r; -0.21 in CPI/r.
The PEY-adjusted AE profile was similar between TPV/r and CPl/r groups, respectively: any grade 3/4 AE: 25.9 vs. 28.9/100 PEY; serious: 20.0 vs. 22.2; AEs resulting in discontinuation: 9.4 vs. 6.8. GI AEs were most common in TPV/r and CPl/r, respectively: diarrhoea: 28.0 vs. 35.1; nausea: 15.7 vs. 19.3; vomiting 9.4 vs. 10.5.
Grade 3/4 ALT/AST elevations and hyperlipidemia were more frequent in TPV/r patients. HTE patients who responded to TPV/r experienced durable responses lasting more than 3 years.
TPV/r response rates were approximately twice those of CPl/r and were markedly higher for those patients who also started a new class of ARV (ENF). The safety profile of TPV/r was similar to previously observed, suggesting long term tolerability among those with initial treatment success.
Introduction
Tipranavir (TPV, Aptivus®) is a second generation protease inhibitor (PI) that exhibits potent activity against multiple PI-resistant HIV-1. TPV/r is effective and well tolerated in patients who have taken more than one PI-based regimen and are infected with HIV-1 that exhibits reduced susceptibility to more than one PI [1-4]. RESIST 1 and 2 are randomized Phase III studies of tipranavir/r (TPV/r) at a dose of 500/200 mg BID or a comparator ritonavir-boosted protease inhibitor (CPl/r), plus optimized background regimen (OBR) in highly treatment experienced (HTE) patients [1,5,6]. Week 24, 48 and 96 efficacy and safety data from RESIST have been presented: at all of these timepoints, treatment with
TPV/r was associated with superior virological and immunological responses when compared to CPI/r [1,5-9]. The RESIST study is ongoing and this poster reports three year (Week 156) data.
Study design
RESIST 1 and 2 enrolled adult patients with HIV infection who fulfilled the following criteria. Full details of the entry criteria are provided in reference
[1]:
⋅ ≥3 consecutive months' experience with 3 classes of antiretroviral (ARV) drugs (NRTIs, NNRTIs, PIs)
⋅ ≥2 PI-based regimens for ≥3 months; one of which was the current treatment regimen
⋅ Viral isolate carrying ≥1 primary protease mutation at 30N, 46I/L, 48V, 50V, 82A/F/L/T, 84V, 90M
⋅ Viral isolate carrying ≦2 mutations at codons 33, 82, 84, 90.
The investigators selected the CPI/r (lopinavir/r, indinavir/r, saquinavir/r or amprenavir/r) and the OBR prior to randomization based on genotypic resistance testing (Figure 1). The use of enfuvirtide (ENF) was allowed; patients were stratified by the planned use of ENF, as well as by the preselected CPI/r.
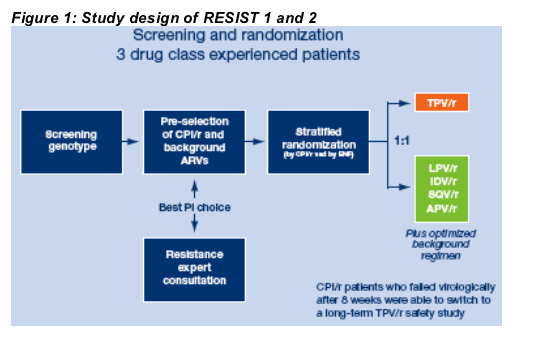
Patients received 500/200 mg BID TPV/r or standard doses of the CPI/r plus approved doses of the components of the OBR.
After Week 8, patients who failed virologically in the CPI/r arm were able to receive TPV/r prior to its regulatory approval via a long term parallel safety study, provided that there was documented evidence that they had been adherent to their study medication.
Treatment response (TR) rates (confirmed VL reduction ≥1 log10 copies/mL at Week 156 without viral rebound [confirmed VL <1 log10 copies/mL below baseline], death or treatment changes), time to treatment failure (TTF), and the proportions of patients with viral loads (VL) <400 and <50 copies/mL at Week 156 were determined. The TTF was analyzed using Cox proportional hazards models or log-rank tests. Analyses were Intent to Treat, Non-Completer equals Failure (ITT NCF) unless otherwise stated.
Results
At Week 156, 218/1483 patients (15.7%) were still taking assigned study medication: 155 were taking TPV/r; and 63 were taking CPI/r. The rate of treatment discontinuation was higher in the CPl/r arm than in the TPV/r arm due to an inferior virologic response rate. Discontinuation was predominantly due to virologic failure: in the TPV/r arm, approximately one quarter (201/746; 26.9%) of patients failed virologically compared to nearly half (352/737; 47.8%) of the CPl/r patients. Overall, the number of TPV/r patient exposure years (PEY) was twice that of the CPl/r patients: 1315.3 vs. 688.5 PEY.
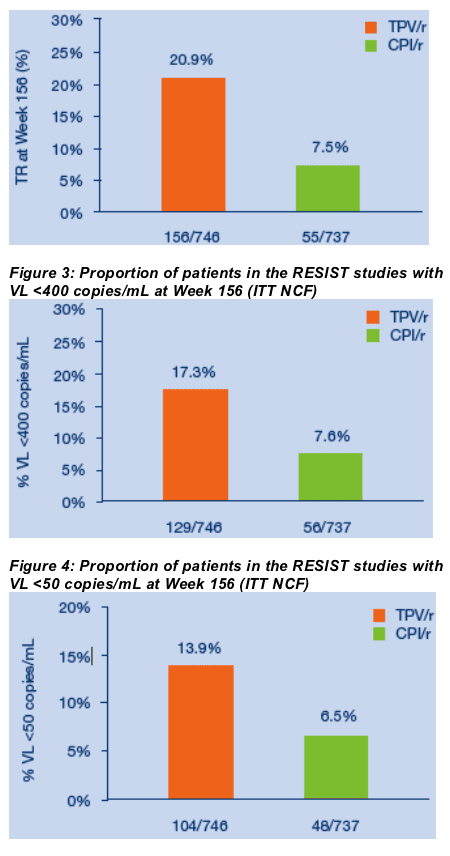
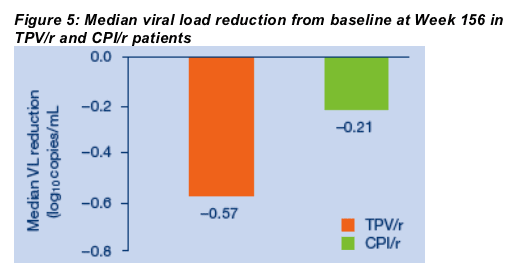
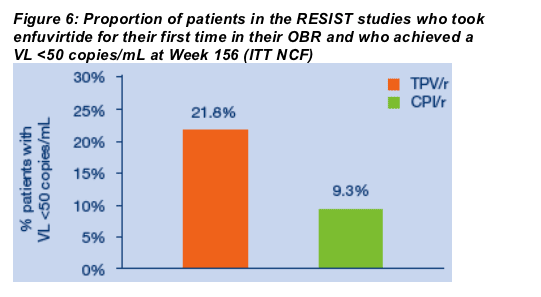
For the overall study population at three years, the proportions of TPV/r patients with VLs <50 copies/mL or <400 copies/mL or with treatment response were approximately twice those achieved by patients taking CPI/r. Treatment response rates at Week 156 were 20.9% (156/746) in the TPV/r arm versus 7.5% (55/737) in the CPI/r arm (ITT NCF) (Figure 2). The proportions of patients who had VLs <400 copies/mL at Week 156 were 17.3% (129/746) and 7.6% (56/737) in the TPV/r and CPIr/r arms, respectively (ITT NCF) (Figure 3). Using the <50 copies/mL assay, 13.9% of TPV/r patients had undetectable VLs at Week 156 as compared to 6.5% of CPI/r patients (ITT NCF) (Figure 4). Median VL decreases from baseline were -0.57 log10 copies/mL in the TPV/r arm and -0.21 log10 copies/mL in the CPI/r arm (Figure 5).
The results of previous analyses demonstrated that patients who added a new class of antiretroviral drug (e.g. enfuvirtide) to their OBR plus TPV/r or CPl/r had improved virological and immunological responses [1,5,6]. At >3 years, TPV/r patients who took enfuvirtide for the first time in their OBR achieved four-fold greater treatment response rates than CPl/r patients: 37.9% vs. 8.2%. In this group of patients, the proportions with VLs <50 copies/mL were 21.8% versus 9.3%, respectively (Figure 6) (ITT NCF).
Figure 2: Week 156 treatment response rates in TPV/r and CPI/r arms
of RESIST studies (ITT NCF)
TR = confirmed VL reduction ≥1 log10 copies/mL at Week 156 without viral rebound [confirmed VL <1 log10 copies/mL below baseline], death or treatment changes
The PEY-adjusted adverse event (AE) profile was similar between the TPV/r and CPl/r groups. Any Grade 3/4 AE occurred in 25.9/100 PEY in the TPV/r arm versus 28.9/100 PEY in the CPI/r arm. Serious AEs occurred at a rate of 20.0/100 PEY in the TPV/r arm versus 22.2/100 PEY in the CPI/r arm. The rate of AEs that resulted in discontinuation of study medication was: 9.4/100 PEY in the TPV/r arm and 6.8/100 PEY in the CPI/r arm. Gastro intestinal (GI) AEs were less common in the TPV/r arm than the CPI/r arm: diarrhea: 28.0/100 PEY in the TPV/r arm vs. 35.1/100 PEY in the CPI/r arm; nausea: 15.7/100 PEY vs. 19.3/100 PEY, respectively; and vomiting 9.4/100 PEY vs. 10.5/100 PEY, respectively. Grade 3/4 ALT/AST elevations (100/737 [13.6%] vs. 22/727 [3%]) and hyperlipidemia (triglycerides: 197/737 [26.7%]) vs. 100/727 [7.7%])) were more frequent in TPV/r patients than in those taking a CPI/r.
References
1. Hicks CB, et al. Lancet 2006; 368(9534): 466-75.
2. Baxter JD, et al. J Virol, 2006; 80(21): 10794-801.
3. Markowitz M, et al. J Acquir Immune Defic Syndr 2007; 45(4): 401-10.
4. Gathe JC, Jr, et al. AIDS Res Hum Retroviruses 2007; 23(2): 216-23.
5. Farthing C, et al. In 46th Interscience Conference on Antimicrobial Agents and
Chemotherapy (ICAAC). 2006. San Francisco. Abs. H-1385.
6. Gazzard B, et al. In 8th International Congress on Drug Therapy in HIV Infection. 2006. Glasgow, UK. Abs. P23.
7. Cahn P, Hicks C, and the RESIST Study Teams. In 10th European AIDS Conference (EACS). 2005. Dublin, Ireland. Abs. PS3/8.
8. Cahn P and the RESIST 2 Study Team. In 7th International Congress on Drug
Therapy in HIV Infection. 2004. Glasgow, UK. Abs. PL14.3.
9. Hicks C. and the RESIST 1 Study Team. In 44th Interscience Conference on
Antimicrobial Agents and Chemotherapy (ICAAC). 2004. Washington. Abs. H-
1137a.
|
| |
|
 |
 |
|
|