 |
 |
 |
| |
Low-Level HIV-1 Viral Load Monitoring in Cerebrospinal Fluid: Possible Correlation to Antiretroviral Drug Penetration
|
| |
| |
Reported by Jules Levin
11th European AIDS Conference, Oct 24-27, 2007, Madrid, Spain
D. McClernon1, S. Letendre2, R. Benjamin6, D. Clifford3, A. Collier4,
B. Gelman5, J. McArthur6, A. McCutchan1,S. Morgello7, I. Grant1,
R. Ellis1, and the CHARTER Group
1GlaxoSmithKline, Research Triangle Park, NC ; 2University of California, San Diego, CA, USA;
3Washington University, St. Louis, MO, USA; 4University of Washington, Seattle, WA, USA;
5University of Texas Medical Branch, Galveston, TX, USA; 6Johns Hopkins University,
Baltimore, MD, USA; 7Mt. Sinai School of Medicine, New York, NY, USA
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is supported by award N01 MH22005 from the National Institutes of Health.
AUTHOR SUMMARY
An enhanced NASBA-based assay detected HIV RNA in half of CSF specimens that were undetectable by the standard assay.
If this reflects HIV replication, then these individuals may be at greater risk for developing brain injury and reduced susceptibility to antiretrovirals.
Detectable HIV RNA in CSF was associated with indicators of better immune competence. Overall, however, the group had advanced disease
(71% AIDS, median nadir CD4 count 138/⊥).
Considering the advanced disease of most of the subjects together with the substantial immune recovery of most (median current CD4 count 421/⊥), the detectable HIV in CSF may reflect ongoing low-level immune reconstitution-related injury, as has recently been described.6,7
Worse antiretroviral penetration was associated with detectable HIV in CSF, particularly in the more clinically relevant subgroup (HIV RNA levels in
plasma below 50 c/mL).
--Among nucleoside RTIs, abacavir and zidovudine were associated with better response than tenofovir. Among protease inhibitors, lopinavir was associated with better response than atazanavir.
--Together, these findings suggest that the effectiveness of ART in the CNS may be improved by switching to better penetrating drugs.
The analysis has important limitations.
--This sample of 125 were selected based on specific criteria. This selection process limits the generalizability of the findings.
--The primary analyses were cross-sectional, which cannot completely control for important time-based measures, such as duration of infection or treatment.
ABSTRACT
Background: HIV Neurocognitive Impairment (HNCI) remains highly prevalent despite the use of combination antiretroviral therapy (ART). ART can reduce HIV RNA in plasma below 50 c/mL but penetrates into the central nervous system in reduced concentrations, which may allow ongoing replication and injury.
The objective of this analysis was to determine the proportion of CSF specimens that have HIV RNA > 2.5 copies (c)/mL among those < 50 c/mL as measured with an ultrasensitive commercial assay.
Methods: 125 CSF specimens with HIV RNA levels < 50 c/mL were selected from 125 participants of the CHARTER study, a North American observational cohort. Specimens were selected to vary potentially influential factors, such as HIV RNA in plasma, blood CD4+ counts, HCV serostatus, and neuropsychological (NP) performance. 2 mL CSF specimens were assayed using the realtime NucliSens EasyQ HIV-1 assay (bioMerieux, Inc.) and software
interpolation capable of quantifying HIV RNA to 2.5 c/mL. RNA data were transformed to an ordinal variable (> or ≦ 2.5 c/mL) and compared with other measures using parametric and nonparametric statistical tests. A CNS Penetration Effectiveness (CPE) Score was calculated as previously described with higher CPE Scores indicating better CNS penetration.
Results:
112 subjects (90%) were taking ART; 40 (32%) had HIV RNA in plasma <50 c/mL; the median CD4 count was 421/⊥ 89 (71%) had been diagnosed with AIDS. 62 (50%) CSF specimens had HIV RNA > 2.5 c/mL.
Detectable levels in CSF were associated with higher HIV RNA in plasma (median 217 vs. 133 c/mL, p=.01) and higher CD4 nadirs (median 180 vs. 107/⊥, p=.009). Detectable levels were not associated with current CD4
counts, AIDS diagnosis, HCV seropositivity, worse NP performance, or duration of regimen.
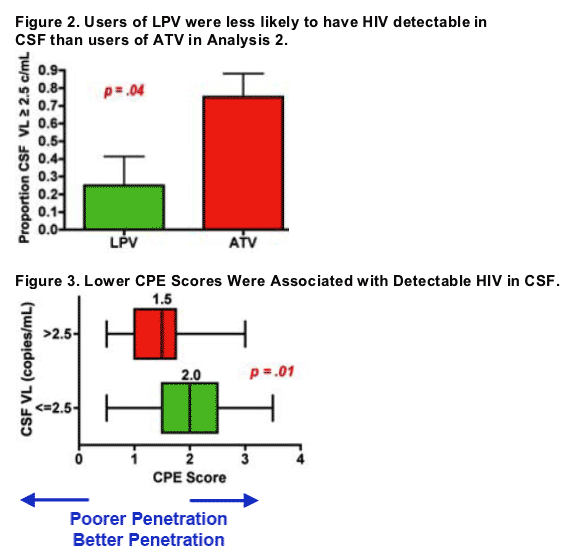
Among the 40 subjects with HIV RNA < 50 c/mL in both CSF and plasma, 17 (42%) had measurable RNA in CSF and this was associated with lower CPE
scores (median 1.5 vs. 2.0, p=.01). Twelve subjects who were taking ART had a second study visit. Three of these individuals had undetectable RNA in CSF at their first visit and detectable RNA at the second. CPE Scores were also lower in these 3 subjects than in the other 9 (median .75 vs. 2, p=.02).
Conclusions:
Nearly half of CSF specimens that had undetectable HIV RNA with an ultrasensitive commercial assay had measurable virus with a more sensitive assay.
Lower ART penetration was associated with detectable HIV RNA in CSF in a clinically relevant subgroup and in the small number of longitudinal specimens.
These analyses indicate that a substantial proportion of individuals have HIV in CSF despite otherwise successful ART, which may explain the occurrence of HNCI in treated individuals.
BACKGROUND
HIV Neurocognitive Impairment (HNCI) remains highly prevalent despite the use of antiretroviral therapy (ART). ART can reduce HIV RNA in plasma below the lower limit of quantitation of commercial assays (50 copies (c)/mL) but replication may persist at low levels, enabling viral adaptation to immunologic and pharmacologic pressures.1,2
Protected compartments, such as the central nervous system (CNS), may be at particular risk for persistent HIV replication because antiretrovirals may not reach therapeutic concentrations there. Two recent studies assessed whether HIV can persist at low levels in the CNS by measuring HIV RNA in CSF using assays with
improved sensitivity. In one study, 8 of 13 (62%) antiretroviral-treated subjects had more than 2 c/mL of HIV in plasma but none surpassed this level in CSF.3 In the second study, 13 of 47 (28%) subjects taking successful ART had more than 2.5 c/mL in CSF.4
We showed last year that individuals who took antiretroviral regimens with worse penetration characteristics were more likely to have HIV RNA levels in CSF above 50 c/mL.5 (Letendre et al., CROI 2006, Abstract 74). The two main objectives of this study were to--
⋅ Determine the proportion of CSF specimens that have HIV RNA levels > 2.5 c/mL among those that have < 50 c/mL with a commercial ultrasensitive assay
⋅ Determine the correlates of HIV RNA levels > 2.5 c/mL, including the effect of antiretroviral penetration.
1. Frenkel LM, et al. J Virol. 77: 5721-30, 2003.
2. Gunthard HF, et al. J Virol. 72: 2422-8, 1998.
3. Yilmaz A, et al. Antivir Ther. 11: 833-7, 2006.
4. Spudich S, et al. J Infect Dis. 194:1686-96, 2006.
5. Letendre S, et al. 13th CROI. Abstract 74, 2006.
6. Nath A and Sacktor N. Curr Opin Neurol. 19: 358-61, 2006.
7. Riedel et al. Nat Clin Pract Neurol. 2: 557-65, 2006.
METHODS
Participants: CHARTER is a North American cohort study based at six sites: Baltimore, Galveston, New York, St. Louis, San Diego, and Seattle. This analysis included HIV-infected individuals from the CHARTER cohort who completed standardized assessments, had successful lumbar punctures, and had HIV RNA levels measured in both plasma and CSF.
Neuropsychological Testing: All participants completed a battery of neuropsychological tests that have demonstrated sensitivity to HIV infection, including measures of working memory, information processing speed, learning and memory, motor skills, verbal fluency, and executive functions. These data were summarized using the Global Deficit Score methodology, which accounts for both the number and severity of deficits evident in an individual's neuropsychological profile (range = 0-5, with higher scores indicating greater levels of impairment) and global impairment ratings.
Laboratory Procedures: HIV RNA were measured in plasma and CSF by the Roche Amplicor® Ultrasensitive assay. CSF specimens were then re-assayed with the real-time NucliSens® EasyQ HIV-1 assay (bioMérieux) capable of quantifying as low as 2.5 c/mL. This validated assay incorporates molecular beacons for detection.
Statistical Analysis: Univariate analyses were performed using Fisher's Exact tests for categorical variables, t-tests for normally distributed continuous variables, or Wilcoxon rank sum tests for skewed continuous variables that were not improved with transformation. HIV RNA levels in CSF and plasma were log10-transformed to improve their distribution.
RESULTS
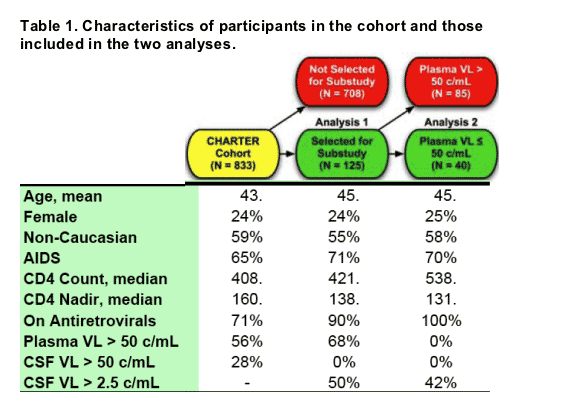
Participant Characteristics
Table 1 summarizes the characteristics of the 125 participants. The analyses were performed in two stages. Analysis 1 included the first measurement for all 125 subjects. 113 subjects (90%) were taking ART; 40 (32%) had HIV RNA in plasma ≦ 50 c/mL; the median CD4 count was 421/⊥ and 89 (71%) had been diagnosed with AIDS. Analysis 2 was limited to subjects who had HIV RNA levels ≦ 50 c/mL in plasma.
Analysis 1 (N = 125, HIV RNA ≦ 50 c/mL in CSF, no limit on HIV RNA in plasma)
HIV was detectable in 62 (50%) CSF specimens. Detectable levels in CSF were associated with higher HIV RNA in plasma (median 2.34 vs. 2.12 log10 c/mL, p=.01), higher CD4 nadirs (median 180 vs. 101/⊥, p=.03), and the absence of an AIDS diagnosis (64% AIDS vs. 79%, p=.04). Detectable levels trended towards associations with higher current CD4 counts (425 vs. 408/⊥, p=.059). Detectable levels were not associated with HCV seropositivity, worse neuropsychological performance, or the duration of the current regimen.
To determine the relationship between the estimated penetration of antiretroviral regimens and detectable HIV in CSF, we calculated the CNS Penetration-Effectiveness Score (CPE Score) for the 90% of subjects taking ART. Subjects who had detectable HIV in CSF trended towards taking regimens that had lower CPE Scores (median 1.5 vs. 2.0, p=.09). We also compared use of the most common drugs within 3 antiretroviral classes.
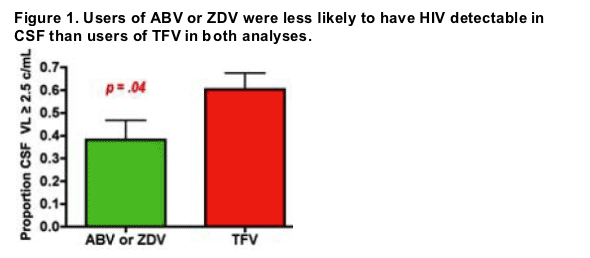
Among nucleoside reverse transcriptase inhibitors (RTIs), subjects who used tenofovir (TFV, n=48) had over twice the odds of having detectable HIV in CSF, compared with use of either abacavir or zidovudine (ABV or ZDV, n=34) (60% vs. 38%, OR 2.46, p=.04, Figure 1). Among non-nucleoside RTIs, detection of HIV in CSF from subjects who used efavirenz (EFV, n=27) did not differ from that of subjects who used nevirapine (NVP, n=14). Among the protease inhibitors, detection of HIV in CSF from subjects who used atazanavir (ATV, n=31) did not differ from that of subjects who used lopinavir (LPV, n=27).
Analysis 2 (N = 40, HIV RNA ≦ 50 c/mL in CSF & Plasma)
HIV was detectable in 17 (42%) CSF specimens. Detectable levels in CSF were
associated with higher nadir CD4 counts (215 vs. 101, p=.04) and the absence of
an AIDS diagnosis (73% vs. 28%, p=.03). Subjects who had detectable HIV in CSF also trended towards having higher current CD4 counts (median 580 vs. 481, p=.06) and global impairment (detectable 100% impaired vs. 59%, p=.07). Detectable levels were not associated HCV seropositivity or duration of the current antiretroviral regimen. Detectable levels in CSF were also associated with lower overall CPE scores (median 1.5 vs. 2.0, p=.01, Figure 3). In drug-specific comparisons, use of TFV (n=15) was associated with detectable HIV
in CSF compared with ABV/ZDV (n=14) (67% vs. 21%, p=.02) as was ATV (n=12) compared with LPV (n=8) (75% vs. 25%, p=.04). EFV (n=9) did not differ from NVP (n=5) (44% vs. 40%, p>.10).
Longitudinal Analysis (N = 12)
Twelve subjects who were taking ART had HIV measured in a second CSF specimen. Three of these individuals had undetectable RNA in CSF at
their first visit and detectable RNA at the second. These subjects had lower CPE Scores than the other 9 (median .75 vs. 2, p=.02).
|
| |
|
 |
 |
|
|