| |
Hepatitis B Virus Treatment in HIV-infected Patients
|
| |
| |
Hepatitis B virus (HBV) infection is common in HIV-infected persons and is associated with increased risk of liver-related morbidity and mortality. Agents available to treat HBV infection in coinfected patients include lamivudine, entecavir, emtricitabine, adefovir, tenofovir, peginterferon alfa, and the recently approved telbivudine. Treatment decisions should take into account a number of factors, including antiretroviral therapy status, HBV genotype, prior experience of lamivudine, and the need to avoid drug resistance in both HIV- and HBV-infected persons. This article summarizes a presentation on treatment and management of HBV infection in HIV-infected patients made by Chloe L. Thio, MD, at the 9th Annual Ryan White CARE Act Clinical Update in Washington, DC. Dr Thio is an Associate Professor of Medicine at The Johns Hopkins University in Baltimore, Maryland. Article from IAS-USA Topics in HIV Medicine, iasusa.org
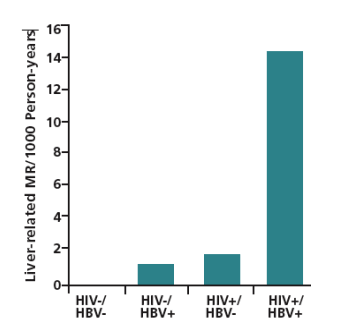
It is estimated that 1 to 1.25 million persons in the United States (US) have hepatitis B virus (HBV) infection. The incidence of infection decreased by 67% between 1990 and 2002, reflecting at least in part the use and efficacy of HBV immunization. However, between 1999 and 2002, incidence rates increased by 5% in men aged 20 to 39 years, and by 20% in men and 31% in women aged 40 years or older (age groups that are also at increased risk of HIV infection). Coinfection with HIV is common. It is estimated that approximately 10% of HIV-infected individuals also have chronic HBV infection. Coinfected patients have increased risks of rapid progression of HBV liver disease, liver-related mortality, antiretroviral therapy-related hepatotoxicity, and hepatic failure as part of an immune reconstitution syndrome. Figure 1 shows the marked increase in liver-related mortality in coinfected patients in a study of 5293 men with or without HIV or HBV infection (Thio et al, Lancet, 2002).
Case 1: Patient With No Prior
Antiretroviral Therapy
A 28-year-old white man is referred for elevated liver function tests. He has a
history of hypertension and review of systems shows fatigue and decreased
appetite. Physical exam shows no jaundice and no right-upper quadrant tenderness or hepatosplenomegaly. Laboratory tests show alanine aminotransferase (ALT) of 126 IU/mL and total bilirubin of 0.6 mg/dL, and the patient is positive for HBV surface antigen (HBsAg). What test is most helpful in initially characterizing the patientfs HBV infection:
1. HBV DNA,
2. Liver biopsy,
3. HBV envelope antigen (HBeAg),
4. 1 and 3, or
5. 1, 2, and 3?
This patient should be tested for both HBV DNA and HBeAg. Acute HBV infection is indicated by an IgM antibody response to HBV core antigen (HBcAg).
Chronic infection is indicated by presence of HBV surface antigen (HBsAg) for more than 6 months. Individuals who have responded to HBV vaccine exhibit anti-HBs antibody, and those with past infection exhibit both anti-HBs antibody and anti-HBc antibody. The patientfs HBsAg-positive status indicates chronic infection. Among patients with chronic infection, active infection is indicated by
HBeAg-positive status, which is often accompanied by ALT greater than 2
times the upper limit of normal, HBV DNA level greater than 1 x 105
copies/mL, 100,000 copies/ml, (about 2 x 104 IU/mL), and evidence of disease on liver biopsy. Patients who are HBeAg-negative also may have active infection, with similar ALT levels and positive liver biopsy but may have lower HBV DNA levels (eg, greater than 104 copies/mL, 10,000 copies/ml, [about 2 x 103 IU/mL]) than HBeAg-positive patients. HBeAg-negative patients without active infection,
"healthy carriers," tend to have normal ALT levels and undetectable, or very low,
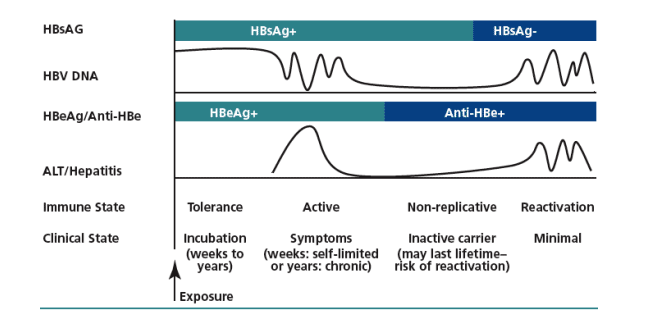
HBV DNA levels and would be unlikely to have significant findings on liver biopsy. Figure 2 provides an idea of how serologic markers, liver function tests, and clinical and immune states are correlated in the natural history of HBV infection. HBV/HIV-coinfected patients who are HBV-inactive carriers require monitoring for HBV DNA and ALT (eg, every 6-12 months) to promptly detect reactivation of HBV infection.
The patient is found to have HIV infection, with a CD4+ cell count of 500/_L.
He is HBsAg-positive, HBeAg-positive, and anti-HBe antibody-negative, has
HBV DNA of 5 * 106 IU/mL, and stage 1 to 2 fibrosis on liver biopsy. What other
tests should be done:
1. Ultrasound or computed tomography (CT) scan and measurement of alpha-fetoprotein,
2. Total hepatitis A virus (HAV) antibody,
3. HBV genotype,
4. 1 and 2, or
5. 1, 2, and 3?
Figure 1. Liver-related mortality rate (MR) per 1000 person-years in 5293 men with (+) or without (-) hepatitis B virus (HBV) or HIV infection. Cohort included 326 men who were HBV surface antigen-positive (HBsAg+). Adapted from Thio et al, Lancet, 2002.
Patients with HBV infection are at increased risk for hepatocellular carcinoma,
which should be ruled out by ultrasound or CT scan and testing for alpha-fetoprotein. They are also at increased risk of more fulminant HAV infection;
HAV antibody should be assessed, and the patient should receive hepatitis A
vaccine if he is antibody-negative.
HBV genotyping is becoming increasingly useful in HBV-disease management, as more is learned about associations of genotype with disease progression (including risk of hepatocellular carcinoma) and response to HBV treatment. For example, in a recent trial comparing peginterferon alfa with peginterferon alfa plus lamivudine in patients without HIV infection, response measured as HBeAg loss occurred in 29% and 44% of patients, respectively, at end of treatment at week 52 (P=.01), with the response rate increasing to 36% in the monotherapy
group and decreasing to 35% in the combination group at end of follow up at
week 78 (P = not statistically significant [NS]; Janssen et al, Lancet, 2005). The study indicated that the addition of lamivudine did not provide benefit and
showed that HBV genotype influenced response at end of follow up. Among all
patients, response at week 78 was observed in 47% of 90 patients with
genotype A, 44% of 23 patients with type B, 28% of 39 patients with type C, and
25% of 103 patients with type D. Types A and D are the most common in the
United States, and there was a statistically significant increase in response for
type A versus type D (odds ratio, 2.4; 95% confidence interval, 1.3-4.6; P=.01) in the study. There currently are no data on association of genotype with response in coinfected patients.
In addition to the findings already noted, genotyping shows the patient to be
infected with HBV genotype A. His plasma HIV RNA level is 12,000 copies/mL.
What treatment should he receive:
1. Lamivudine,
2. Entecavir,
3. Tenofovir,
4. Peginterferon alfa, or
5. 2 or 4?
US Food and Drug Administration (FDA)- approved therapies for HBV infection
consist of: the L-nucleoside analogues, lamivudine, telbivudine, and entecavir;
the nucleotide analogue, adefovir; and peginterferon alfa. Agents with activity
against HBV that are available but not FDA-approved include the nucleoside analogue emtricitabine, and the nucleotide analogue tenofovir. Lamivudine, emtricitabine, and tenofovir have intrinsic anti- HIV activity. Adefovir, a forerunner of tenofovir, also has anti-HIV activity but is given at lower doses in HBV infection
than those associated with anti-HIV activity. Adefovir is not used for HIV
treatment because doses with anti-HIV activity were associated with severe renal
toxicity. Peginterferon alfa has some anti-HIV activity but is not associated
with HIV resistance. Given the patientfs HIV RNA and CD4+ cell count, he can
begin treatment for HBV infection before antiretroviral therapy for HIV is
considered. To avoid the emergence of HIV resistance that would arise with
suboptimal suppression of viral replication and compromise future treatment,
the agents with intrinsic anti-HIV activity should not be used to treat HBV
infection. Of the choices listed, one option for the patient is peginterferon
alfa; however, it has yet to be assessed in patients with coinfection. Entecavir
is also an option since published data do not show evidence of anti-HIV activity.
Other options, which are not listed, include initiating antiretroviral therapy early or adefovir.
Figure 2. Natural history of hepatitis B virus (HBV) infection. HBsAg indicates HBV surface antigen; HBeAg, HBV envelope antigen; anti-HBe, antibody to HBe; ALT, alanine aminotransferase; +, positive; -, negative.
Case 2: Patient On Antiretroviral
Therapy
A 42-year-old HIV-infected man presents with elevated liver enzymes. He has
received zidovudine, lamivudine, and efavirenz for the past 3 years and has a
CD4+ cell count of 350/_L and plasma HIV RNA level below 50 copies/mL. He is
HBsAg-positive and HBeAg-negative, with HBV DNA of 3.6 * 105 IU/mL. What
should you do with his medications:
1. Replace zidovudine with tenofovir,
2. Continue current medications,
3. Add entecavir,
4. Replace lamivudine with emtricitabine,
or
5. None of the above?
The patient has been receiving lamivudine for 3 years as part of a antiretroviral
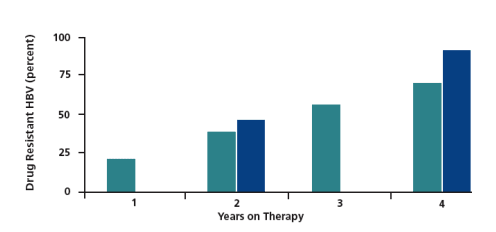
therapy regimen that remains effective in suppressing HIV replication. HBV resistance to lamivudine develops more rapidly in coinfected patients than in HBV-monoinfected patients (Figure 3), with approximately 90% of coinfected patients having HBV resistance after 4 years of treatment. The emergence of resistance is clinically evidenced by increases in transaminases. For example, in one study ALT flares (greater than 3 times the upper limit of normal) showed statistically significant increases in patients with lamivudine resistance, as well as statistically significant increases as the duration of presence of lamivudine resistance lengthened.
Hepatitis flares occurred in about 20% of patients with no evident lamivudine
resistance, about 35% of those with presence of resistance mutations for
less than 1 year, and about 75% of those with mutations present for more
than 4 years. Hepatic decompensation and liver-related adverse events increased after 4 years of lamivudine resistance (Lok et al, Gastroenterology, 2003). In this patient, the lamivudine is needed for his HIV infection. Although
data exist suggesting that continuing lamivudine despite resistance might
still benefit the patient in terms of continued reduction in HBV DNA and maintained loss of HBeAg, growing evidence, on hepatitis flares and the association of such flares with worsened liver disease, argue against continuing lamivudine alone in the setting of resistance. The patientfs medications need to be changed to provide additional anti-HBV activity. Among potential drugs that may be substituted or added are adefovir, tenofovir, and entecavir. Adefovir does
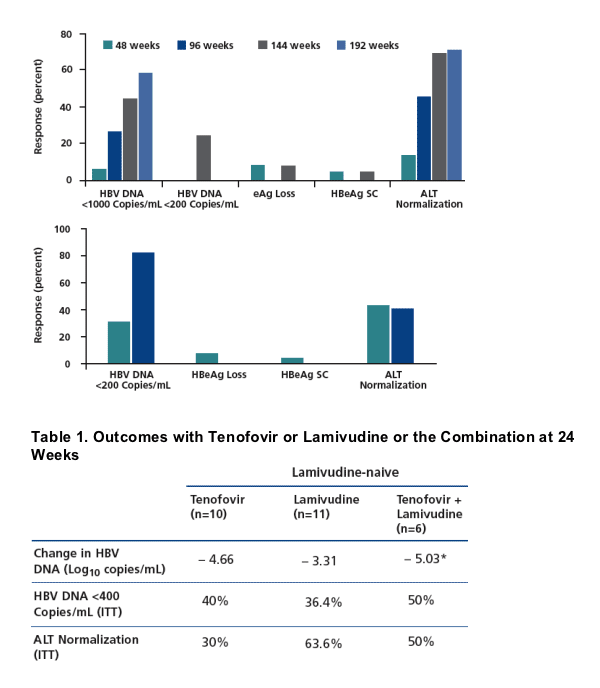
not appear to be as effective in treating HBV infection as tenofovir. In a cohort of
35 coinfected patients with lamivudine-resistant HBV, 48-week outcomes with
adefovir treatment (10 mg/d) were relatively poor, including reduction in HBV
DNA to below 1000 copies/mL in 6%, HBeAg loss in 8.6%, and ALT normalization in 14%, although responses appeared to improve over longer durations (Figure 4; Benhamou et al, J Hepatol, 2006). There is a concern that adefovir treatment might result in HIV that is resistant to tenofovir; no evidence of resistance was found in the above study or in other limited experience with adefovir in this setting.
AIDS Clinical Trial Group (ACTG) study 5127 was performed to demonstrate
noninferiority of tenofovir 300 mg compared with adefovir 10 mg in reducing
HBV DNA in 52 coinfected patients on stable antiretroviral therapy, who had
HBV DNA levels above 100,000 copies/mL and HIV RNA levels below
10,000 copies/mL (Peters et al, CROI, 2005 and Hepatology, 2006). The time-weighted average reductions in HBV DNA at 48 weeks with tenofovir versus
adefovir were 4.03 versus 3.12 log10 on intent to treat analysis and 4.76 versus
3.48 log10 on an as-treated analysis, respectively. In a cohort study of tenofovir
in coinfected patients with lamivudine-resistant HBV, 1-year outcomes were
better than those reported in the adefovir cohort study, including greater proportions of patients with HBV DNA levels reduced to below 200 copies/mL and greater proportions of patients with ALT normalization (Figure 4; Benhamou et al, Hepatology, 2006). In a trial comparing entecavir with continued lamivudine
for 24 weeks in 68 coinfected patients with lamivudine-resistant HBV, mean
HBV DNA of 9.13 log10 copies/mL and mean HIV-1 RNA of 2 log10 copies/mL,
entecavir (n=51) reduced HBV DNA to below 300 copies/mL in 6% compared
with 0% with lamivudine. The mean decline in HBV DNA was 3.65 log10
copies/mL with entecavir compared with an increase of 0.11 log10 copies/mL
with lamivudine (entecavir package insert).
One study evaluated the combination of lamivudine with tenofovir in
HBV-monoinfected patients. Patients without prior lamivudine exposure had
a significantly greater reduction in HBV DNA with the combination therapy
than with lamivudine alone (Table 1; Nelson et al, CROI, 2006). Among
lamivudine-experienced patients, both the combination therapy and tenofovir
alone significantly reduced HBV DNA compared with lamivudine, with the
combination appearing to improve other outcomes as well, despite the prior lamivudine exposure.
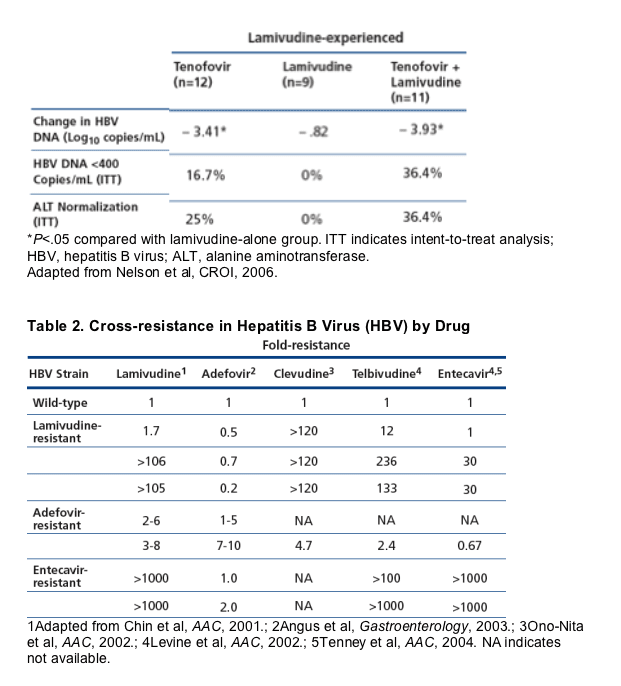
There are few data on HBV resistance to adefovir, tenofovir, and entecavir
(Borroto-Esoda et al, J Hepatol, 2006; Colonno et al, J Hepatol, 2005). Data on
adefovir indicate rates of resistance of 3% in year 2 of treatment, 11% in year
3, 18% in year 4, and 29% in year 5 among mainly HBeAg-negative patients
without HIV infection. Resistance has not been observed in patients continuing
lamivudine despite lamivudine resistance and was not observed in any of 29
coinfected patients over 144 weeks of monitoring. Resistance to entecavir was
observed in 9% of patients at 2 years and occurs mainly in the setting of lamivudine-resistant virus. Entecavir resistance is infrequent in the setting of wild-type HBV. Case reports from Australia now confirm several cases of entecavir resistance in lamivudine-naive, monoinfected patients (Colonno et al, Hepatology, 2006). [note from Jules Levin: entecavir resistance after 3 years followup was reported at AASLD 2006 at <1%. Resistance in treatment-naives might be due to LAM-resistance transmitted by sex or IDU or other means of HBV treansmission]. Entecavir shares 2 of its 4 identified HBV resistance mutations with lamivudine. Table 2 shows cross-resistance among lamivudine, entecavir, adefovir, telbivudine, and the investigational L-nucleoside analogue, clevudine. Resistance to lamivudine is associated with resistance to other L-nucleoside analogues, raising the issue of losing class activity in the presence of lamivudine resistance. Data such as these indicate that the entecavir dose should be increased from 0.5 mg to 1 mg when lamivudine-resistant virus is present. Adefovir-resistant virus also appears to reduce susceptibility to most of the L-nucleoside analogues.
Given the above considerations, a likely option for the patient is to replace zidovudine with tenofovir to maintain anti-HIV efficacy and provide anti-HBV activity. The addition of entecavir might also be considered, but the likelihood of lamivudine resistance in the patient suggests risk for entecavir resistance as well.
Treatment Strategies
Treatment of HBV alone
If HBV infection is to be treated without concurrent antiretroviral therapy (eg, in
patients who have not yet started antiretroviral therapy), drugs that are
not active against HIV should be used to prevent development of drug-resistant
HIV. Peginterferon alfa is an option; it may be most useful in patients who are
HBeAg-positive, have HBV genotype A, and have elevated ALT. Adefovir is
another option. Published data do not show activity of entecavir against HIV,
so that is another option.
Treatment of HBV and HIV
In patients receiving antiretroviral therapy, treatment for HBV infection should
occur only with maximally suppressive antiretroviral therapy. In lamivudine-naive
patients, the first-line therapy consists of tenofovir plus either lamivudine
or emtricitabine with preference given to emtricitabine since it is coformulated
with tenofovir into 1 pill. Other approaches that may be considered include entecavir with or without tenofovir or peginterferon alfa treatment. In lamivudine-experienced patients, the preferred option is the addition of tenofovir to ongoing lamivudine. If this is not possible, other options might include use of entecavir at the 1-mg dose (to compensate for likelihood of reduced susceptibility due to lamivudine resistance) or, in some instances, the addition of adefovir (if sparing of tenofovir is desired), although there is concern regarding efficacy and toxicity with this option.
Monitoring and Duration of Treatment
In patients starting treatment for HBV infection, HBV DNA and liver function
tests should be performed every 3 months for the first year and every 3 to
6 months thereafter, provided resistance is not suspected. Patients who are
HBeAg-positive should be monitored for HBeAg status and anti-HBe antibody
status on the same schedule. Treatment is lifelong in patients who are HBeAg-positive. Those HBeAg-positive patients who seroconvert to HBeAg-negative and
anti-HBe positive should continue treatment for at least 6 months to determine
if seroconversion is stable. [note from Jules Levin: in HIV-negative HBeAg-positive patients who setoconvert there is a the possibility of discontinuing HBV therapy]. Duration of treatment with nucleoside or nucleotide
analogues in patients who begin therapy with HbsAg-negative status is probably
lifelong. The optimal duration of treatment with peginterferon alfa in HBeAg-negative patients remains unclear. Treatment should continue for more than
12 months since relapse is virtually universal in patients treated for less than 12
months. The recommended duration of treatment in HBeAg-positive patients is
12 months.
Summary
Treatment of HBV infection should be considered in HIV-infected persons.
HBV resistance occurs to single-agent therapy, but resistance rates appear to
vary; cross-resistance may also occur. The treatment plan should be individualized based on the need for treatment of HIV infection and prior lamivudine therapy, with a primary objective of preventing emergence of drug-resistant HBV and HIV. More potent agents are needed, and combination therapy needs further investigation.
Figure 4. Top: Outcomes of adefovir treatment in observational cohort of 35 patients with lamivudine-resistant hepatitis B virus (HBV) ; n=31 at 48 weeks and n=29 at 144 weeks. Adapted from Benhamou et al, J Hepatol, 2006. Bottom: Outcomes of tenofovir treatment for 12 months in observational cohort of 65 patients with lamivudine-resistant HBV. Adapted with permission from Benhamou et al, Hepatology, 2006. SC indicates seroconversion; ALT, alanine aminotransferase; eAg, envelope antigen; HBeAg, HBV envelope antigen; + positive; -, negative.
Suggested Reading
Benhamou Y, Bochet M, Thibault V, et al. Longterm incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302-1306.
Benhamou Y, Fleury H, Trimoulet P, et al. Antihepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548-555.
Benhamou Y, Thibault V, Vig P, et al. Safety and efficacy of adefovir dipivoxil in patients infected with lamivudine-resistant hepatitis B and HIV-1. J Hepatol. 2006;44:62-67.
Borroto-Esoda K, Arterbrun S, Snow A, et al. Final analysis of the virological outcomes and resistance during 5 years of adefovir dipivoxil monotherapy in HBeAg-negative patients. J Hepatol. 2006;44:S179-S180.
Bristol-Myers Squibb. Entecavir [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2005.
Chang TT, Lai CL, Chien RN, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004; 19:1276-1282.
Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology. 2006;44:1656-1665.
Colonno RJ, Rose RE, Levine SM, et al. Entecavir (ETV) resistance is not observed in nucleosidenaive subjects and is observed infrequently by week 48 in lamivudine-refactory subjects with chronic HBV infection. J Hepatol. 2005;42:573.
Dore GJ, Cooper DA, Barrett C, Goh LE, Thakrar B, Atkins M. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis. 1999;180:607-613.
Janssen HL, Van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis
B: a randomised trial. Lancet. 2005;365:123-129.
Liaw YF, Leung NW, Chang TT, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180.
Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722.
Nelson M, Bhagani S, Fisher M, et al. A 48-week study of tenofovir or lamivudine or a combination of tenofovir and lamivudine for the treatment of chronic hepatitis B in HIV/HBV co-infected individuals. [Abstract 831.] 13th Conference on Retroviruses and Opportunistic Infections. February 5-8, 2006; Denver, Colorado.
|
|
| |
| |
|
|
|