| |
HIV Type 1 Chemokine Coreceptor Use among Antiretroviral-Experienced Patients Screened for a Clinical Trial of a CCR5 Inhibitor: AIDS Clinical Trial Group A5211
|
| |
| |
Clinical Infectious Diseases Jan 17 Epub 2007;44:000
Timothy J. Wilkin,1 Zhaohui Su,2 Daniel R. Kuritzkes,3 Michael Hughes,2 Charles Flexner,5 Robert Gross,7 Eoin Coakley,8 Wayne Greaves,9 Catherine Godfrey,6 Paul R. Skolnik,4 Joseph Timpone,10 Benigno Rodriguez,11 and Roy M. Gulick1
1Division of International Medicine and Infectious Diseases, Weill-Cornell Medical College, New York, New York; 2Department of Biostatistics, Harvard School of Public Health, 3Section of Retroviral Therapeutics, Brigham and Women's Hospital and Division of AIDS, Harvard Medical School, and 4Center for HIV/AIDS Care and Research, Boston University School of Medicine, Boston, Massachusetts; 5Pharmacology and Molecular Sciences and International Health, Johns Hopkins University, Baltimore, and 6Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; 7Center for Clinical Epidemiology and Biostatistics and Division of Infectious Diseases, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania; 8Monogram Biosciences, South San Francisco, California; 9Schering-Plough Research Institute, Kenilworth, New Jersey; 10Division of Infectious Diseases, Georgetown University School of Medicine, Washington, D.C.; and 11Division of Infectious Diseases, University Hospital of Cleveland, Ohio
study data "suggests that CD4+ cell count alone cannot reliably predict coreceptor use and that the use of specialized assays to determine coreceptor use may be necessary before the initiation of CCR5 inhibitors....Our study found that, in our treatment-experienced population, subjects with X4 virus appeared to have CD4+ cell counts similar to those of subjects in the R5 virus group, and they had lower plasma HIV-1 RNA levels than either the R5 virus group or the D/M virus group....data suggest that the poor prognosis associated with a syncytium-inducing phenotype may be associated with the D/M virus rather than with virus that uses CXCR4 only....In conclusion, we have observed a high prevalence of D/M viral populations in a highly treatment-experienced population screened for a clinical trial of a CCR5 inhibitor. In this group, lower CD4+ cell counts were associated with a higher prevalence of D/M virus, although D/M virus was common among patients at all CD4+ cell count strata. These results suggest the importance of assessing coreceptor phenotype before use of CCR5 inhibitors".
(See the editorial commentary by Melby)
ABSTRACT
Background. Chemokine coreceptor use impacts both the natural history of human immunodeficiency virus type 1 (HIV-1) disease and the potential use of a new class of antiretroviral agents, the CCR5 inhibitors.
Methods. We analyzed HIV-infected patients who were screened for participation in Acquired Immunodeficiency Syndrome (AIDS) Clinical Trial Group protocol A5211, a phase 2b study of the investigational CCR5 inhibitor vicriviroc involving antiretroviral-experienced subjects. Screening CD4+ cell count, HIV-1 plasma RNA level, HIV-1 genotype, and chemokine coreceptor use phenotype were determined. The univariate and multivariate association of subject characteristics with coreceptor use was assessed by logistic regression.
Results.
Coreceptor use was determined for 391 subjects:
--197 (50%) had virus that used the CCR5 coreceptor (the R5 group),
--176 (46%) had dual-tropic or mixed HIV-1 populations that used both CCR5 and CXCR4 coreceptors (the D/M group), and
--16 (4%) had virus that used the CXCR4 coreceptor (the X4 group). --The D/M group had a significantly lower median CD4+ cell count than the R5 virus group (103 cells/uL vs. 170 cells/uL; P < .001).
No other characteristics were independently associated.
--Among 118 subjects who entered A5211 having R5 virus, 12 (10%) had D/M virus according to the results of a second coreceptor test conducted prior to starting treatment with the study drug.
Conclusions. Infection with dual-tropic or mixed HIV-1 populations that use both CCR5 and CXCR4 is common among highly treatment-experienced patients, but infection with virus using CXCR4 alone is uncommon. Subjects in the D/M group had significantly lower CD4+ cell counts than subjects in the R5 group. Evaluating coreceptor use will be important in the clinical development of CCR5 and CXCR4 inhibitors.
Introduction
HIV-1 entry into CD4+ cells is a multiple-step process [1]. First, the external envelope glycoprotein (gp120) of HIV-1 binds to the CD4 receptor on the surface of the CD4+ cell. Subsequently, a conformational change in gp120 allows interaction with a chemokine coreceptor (either CCR5 or CXCR4). Binding of a CD4 receptor and a chemokine coreceptor leads to conformational changes in gp41, followed by fusion of the HIV-1 membrane and the host cellular membrane and the release of the viral core into the cytoplasm.
In the early 1990s, it was noted that the presence of HIV-1 capable of inducing syncytium formation in MT-2 cells was associated with an increased risk of clinical progression to AIDS and death [2]. As the understanding of HIV-1 entry evolved, it became clear that the switch from a non-syncytium-inducing phenotype to a syncytium-inducing phenotype reflected a change in chemokine coreceptor use. The non-syncytium-inducing phenotype correlated with the use of CCR5 as the requisite chemokine receptor for HIV-1 entry, and the syncytium-inducing phenotype correlated with the use or partial use of the CXCR4 chemokine receptor [3].
HIV-1 that uses CCR5 exclusively (R5 virus) predominates early in the course of HIV-1 infection [4, 5]. HIV-1 that uses CXCR4 exclusively (X4 virus) has been found in <1% of antiretroviral-naive HIV-1-infected subjects [4, 5]. Some variants of HIV-1 can use either coreceptor (dual-tropism), and mixed viral populations can occur in a given patient (such patients can be classified as having D/M virus) [6].
CCR5 inhibition is an attractive target for antiretroviral drug development, and several CCR5 inhibitors have entered clinical trials and have established short-term antiretroviral activity [7, 8]. With their novel mechanism of action, CCR5 inhibitors should have antiretroviral activity for HIV-1-infected patients with uncontrolled viremia and few other treatment options (e.g., patients with multidrug-resistant virus). However, these compounds are most likely to be effective if the individual is infected with R5 virus. These compounds have no effect on X4 virus [9]. We sought to describe the epidemiology of and identify the demographic and clinical characteristics associated with chemokine coreceptor use among antiretroviral-experienced subjects who were screened for eligibility in a phase 2b study of a CCR5 inhibitor.
RESULTS
Four hundred and six individuals were screened for A5211, and 391 (96%) had an available coreceptor phenotype result. Virus that used CCR5 only (R5 virus) was found in 197 (50%) of the subjects, dual-tropic virus or a mixed viral population (D/M virus) was found in 178 (46%), and virus that used only CXCR4 (X4 virus) was found in 16 (4%). Of the 197 subjects with R5 virus who were screened for A5211, 118 were eligible for and subsequently entered the trial; the remaining 79 subjects were excluded from the study on the basis of other study entry criteria (e.g., HIV-1 RNA level or receipt of concomitant medications) or withdrew consent. At study entry, 12 (10%) of 118 subjects were classified as belonging to the D/M group on additional testing; these tests were performed a median of 37 days after the screening evaluation (maximum interval, 42 days).
Univariate and multivariate associations of demographic and clinical characteristics with coreceptor use (R5 group vs. D/M group) are shown in table 1. On univariate analysis, the median age was higher for the R5 group than for the D/M group (46 years vs. 44 years; P = .04). White subjects were significantly more likely to have R5 virus than were black subjects (54% vs. 39%; P = .03). Plasma HIV-1 RNA level was significantly lower for subjects with R5 virus, compared with the D/M group (4.5 log10 copies/mL vs. 4.7 log10 copies/mL; P = .03). The median CD4+ cell count at the time of screening was significantly lower for subjects in the D/M group, compared with subjects in the R5 group (103 cells/L vs. 170 cells/L; P < .001). On multivariate analysis, only the screening CD4+ cell count remained significantly associated with coreceptor use. The relationship of screening CD4+ cell count to coreceptor use is shown in more detail in figure 1. The proportion of subjects with R5 virus decreased with lower CD4+ cell counts. For example, 71% of subjects with a CD4+ cell count 350 cells/uL had R5 virus, compared with only 39% of subjects with a CD4+ cell count <50 cells/uL.
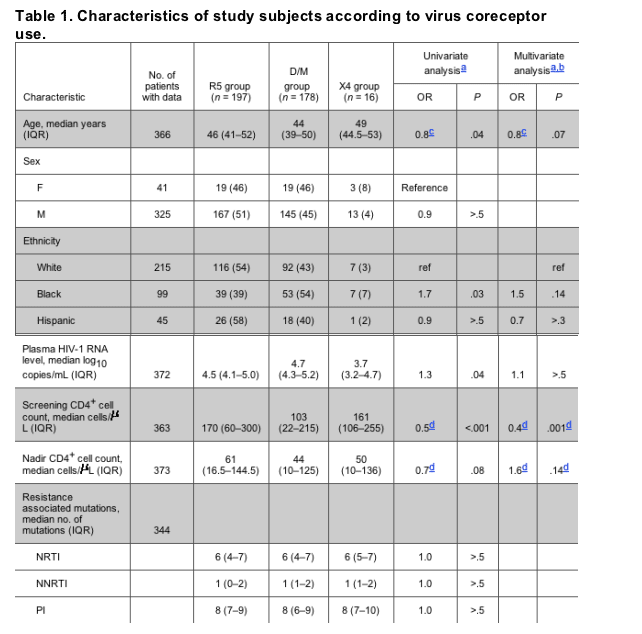
Figure 1. Coreceptor use according to patient screening CD4+ cell count in cells/uL. The proportion of participants in each CD4+ cell count stratum with a given coreceptor-use classification is shown. Screening CD4+ cell count data were not available for 28 participants (including 15 patients in the R5 group and 13 in the D/M group). D/M, dual-tropic or mixed HIV-1 populations using both the CCR5 and CXCR4 coreceptors; R5, virus using the CCR5 coreceptor; X4, virus using the CXCR4 coreceptor.
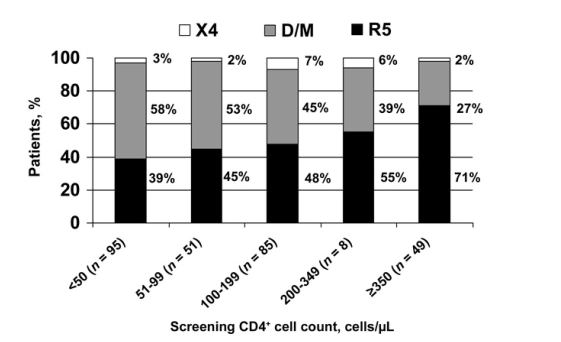
There were 16 subjects with X4 virus only. They had a median plasma HIV-1 RNA level (3.7 log10 copies/mL) that was significantly lower than that of subjects with R5 virus (4.5 log10 copies/mL; P = .002) and of subjects with D/M virus (4.7 log10 copies/mL; P < .001). The median screening CD4+ cell count for subjects with X4 virus (161 cells/L) was not significantly different than that of subjects with R5 virus (170 cells/uL) or D/M virus (103 cells/uL). No other characteristics were found to be independently associated with the presence of X4 virus only.
DISCUSSION
Assessment of coreceptor use may be a critical determinant of eligibility for treatment with the CCR5 and CXCR4 receptor inhibitors currently in clinical development. In this cross-sectional analysis of highly treatment-experienced subjects, we found that one-half of the subjects had virus that used only CCR5 and approximately one-half of the subjects were infected with D/M virus that used CCR5 and CXCR4. Virus that only used CXCR4 was uncommon (found in only 4% of subjects). D/M virus was more common among subjects with lower CD4+ cell counts. However, the prevalence of D/M or X4 virus was relatively common, even among those subjects with higher CD4+ cell counts; the prevalence was 45% among subjects with a CD4+ cell count of 200-350 cells/uL and 29% among subjects with a CD4+ cell count >350 cells/L. This observation suggests that CD4+ cell count alone cannot reliably predict coreceptor use and that the use of specialized assays to determine coreceptor use may be necessary before the initiation of CCR5 inhibitors.
We found a higher overall prevalence of D/M virus than did earlier studies involving antiretroviral-naive subjects (18%-19%) [4, 5] or subjects with limited treatment experience (22%-28%) [5, 10]. Our results were similar to the rates of coreceptor use that were found among highly treatment-experienced subjects enrolled in the T-20 versus Optimized Regimen Only (TORO) 1 and TORO 2 studies, which were large phase 3 trials of enfuvirtide [11]. Our population differed from that of the TORO studies in that it included a higher proportion of nonwhite subjects [12, 13] and had an older median age, a higher median CD4+ cell count, and a lower median plasma HIV-1 RNA level. We were also able to examine the relationship of nadir CD4+ cell count to coreceptor use. All of these studies found a similar relationship between coreceptor use and concurrent CD4+ cell count (a higher prevalence of D/M virus was associated with a lower median CD4+ cell count). However, D/M virus was more common among patients in a given CD4+ cell count stratum in our highly treatment-experienced population and in the TORO population than was seen in the studies involving subjects with limited or no treatment experience. This is most likely because many treatment-experienced subjects had a nadir CD4+ cell count that was much lower than the screening CD4+ cell count in our study. D/M virus that was emerging at the time of these low nadir CD4+ cell counts may have persisted despite the subsequent partial immune reconstitution induced by treatment.
This hypothesis was supported by a recent study that compared coreceptor use in a treatment-experienced cohort and a treatment-naive cohort [14]. This study found an increased prevalence of D/M virus in the treatment-experienced group that was largely explained by differences in the nadir CD4+ cell count. However, X4 use may emerge as a result of antiretroviral treatment itself. Delobel et al. [15] described the emergence of X4-using variants in PBMCs in 11 of 23 subjects receiving antiretroviral therapy despite complete virologic suppression.
The natural history of subjects with HIV-1 using only CXCR4 is not well described. Our study found that, in our treatment-experienced population, subjects with X4 virus appeared to have CD4+ cell counts similar to those of subjects in the R5 virus group, and they had lower plasma HIV-1 RNA levels than either the R5 virus group or the D/M virus group. Similar results were found among subjects enrolled in the TORO 1 and TORO 2 studies [11]. These data suggest that the poor prognosis associated with a syncytium-inducing phenotype may be associated with the D/M virus rather than with virus that uses CXCR4 only. The lower plasma HIV-1 RNA level may be attributable to a depletion of target cells for X4 virus (i.e., naive and resting T cells). In vitro, productively infected naive T cells produce a smaller number of virions, compared with memory T cells (the target for R5 virus), which could lead to lower plasma HIV-1 RNA levels when X4 virus predominates [16].
There are several limitations to this study. The coreceptor use phenotype testing method that was used in this study cannot distinguish dual-tropic virus from mixed viral populations. It is possible that the association of the clinical and demographic variables with coreceptor use would be different when analyzing these categories separately. There was likely some misclassification of subjects, because this assay tests only for major HIV-1 variants circulating in the plasma [6]. Some of the subjects classified as having X4 virus may have had low-level R5 or dual-tropic variants that were not detected by this assay. Ten percent of subjects in the R5 group who subsequently entered the A5211 study were found to have D/M virus on additional coreceptor use testing. Given the relatively short period between evaluations, this change in classification likely represents variability of the assay in testing samples with low-level dual-tropic or X4-using variants, rather than a true change in coreceptor use.
In conclusion, we have observed a high prevalence of D/M viral populations in a highly treatment-experienced population screened for a clinical trial of a CCR5 inhibitor. In this group, lower CD4+ cell counts were associated with a higher prevalence of D/M virus, although D/M virus was common among patients at all CD4+ cell count strata. These results suggest the importance of assessing coreceptor phenotype before use of CCR5 inhibitors.
|
|
| |
| |
|
|
|