| |
Rosuvaststin Improves Lipids & Slows Progression in Low Risk Group with Sub-Clinical Atherosclerosis
|
| |
| |
"Effect of Rosuvastatin on Progression of Carotid Intima-Media Thickness in Low-Risk Individuals With Subclinical Atherosclerosis'
The METEOR Trial
John R. Crouse III, MD; Joel S. Raichlen, MD; Ward A. Riley, PhD; Gregory W. Evans, MA; Mike K. Palmer, PhD; Daniel H. O'Leary, MD; Diederick E. Grobbee, MD, PhD; Michiel L. Bots, MD, PhD; for the METEOR Study Group
Departments of Medicine (Dr Crouse), Neurology (Dr Riley), and Public Health Sciences (Mr Evans), Wake Forest University, Winston-Salem, NC; AstraZeneca, Wilmington, Del (Dr Raichlen); AstraZeneca, Macclesfield, England (Dr Palmer); Caritas Carney Hospital, Boston, Mass (Dr O'Leary); and Department of Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (Drs Grobbee and Bots).
The METEOR study was funded by AstraZeneca.
JAMA. March 26, 2007;297
"...these participants were clinically at low risk, although their intima-media thickness criteria indicated the presence of subclinical atherosclerosis... rosuvastatin lowered LDL-C concentration by 48.8% and raised HDL-C concentration by 8.0% compared with baseline concentrations.... Rosuvastatin did not result in statistically significant regression of CIMT. Given the intima-media thickness inclusion criteria, the participants did not have advanced atherosclerosis and so it may have been optimistic to expect rosuvastatin to cause regression. We chose low-risk individuals with only mild atherosclerosis for the study to justify the use of a placebo group. In contrast to the significant progression of atherosclerosis in the placebo group, no significant progression was observed in the rosuvastatin group. These results indicate that even in this low-risk, middle-aged population with mild atherosclerosis, cholesterol management with rosuvastatin slows the progression of CIMT compared with placebo over a 2-year period. This suggests that rosuvastatin can affect even small increases of CIMT associated with early abnormalities of arterial (presumably intimal) structure. In comparison, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID),6 which included patients with more advanced atherosclerosis, treatment with a 40-mg dose of rosuvastatin for 2 years resulted in regression of coronary atherosclerosis compared with baseline, as assessed by intravascular ultrasound. Presumably, the presence of more advanced disease in ASTEROID allowed detection of regression that was not observed in METEOR. Although a reduction in clinical events by rosuvastatin has not yet been demonstrated, increased CIMT has been linked to increased cardiovascular risk in asymptomatic adults30-32 and a reduced progression of CIMT in statin trials is congruent with a reduction in cardiovascular events... Larger, longer-duration randomized trials focused on clinical events are needed to determine the practice implications of these findings."
ABSTRACT
Context Atherosclerosis is often advanced before symptoms appear and it is not clear whether treatment is beneficial in middle-aged individuals with a low Framingham risk score (FRS) and mild to moderate subclinical atherosclerosis.
Objective To assess whether statin therapy could slow progression and/or cause regression of carotid intima-media thickness (CIMT) over 2 years.
Design, Setting, and Participants Randomized, double-blind, placebo-controlled study (Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin [METEOR]) of 984 individuals, with either age (mean, 57 years) as the only coronary heart disease risk factor or a 10-year FRS of less than 10%, modest CIMT thickening (1.2-<3.5 mm), and elevated LDL cholesterol (mean, 154 mg/dL); conducted at 61 primary care centers in the United States and Europe between August 2002 and May 2006.
Intervention Participants received either a 40-mg dose of rosuvastatin or placebo.
Main Outcome Measures Rate of change in maximum CIMT (assessed with B-mode ultrasound) for 12 carotid sites; changes in maximum CIMT of the common carotid artery, carotid bulb, and internal carotid artery sites and in mean CIMT of the common carotid artery sites. CIMT regression was assessed in the rosuvastatin group only.
Results Among participants in the rosuvastatin group, the mean (SD) baseline LDL cholesterol level of 155 (24.1) mg/dL declined to 78 (27.5) mg/dL, a mean reduction of 49% (P<.001 vs placebo group). The change in maximum CIMT for the 12 carotid sites was -0.0014 (95% CI, -0.0041 to 0.0014) mm/y for the rosuvastatin group vs 0.0131 (95% CI, 0.0087-0.0174) mm/y for the placebo group (P<.001). The change in maximum CIMT for the rosuvastatin group was -0.0038 (95% CI, -0.0064 to -0.0013) mm/y for the common carotid artery sites (P<.001), -0.0040 (95% CI, -0.0090 to 0.0010) mm/y for the carotid bulb sites (P<.001), and 0.0039 (95% CI, -0.0009 to 0.0088) mm/y for the internal carotid artery sites (P = .02). The change in mean CIMT for the rosuvastatin group for the common carotid artery sites was 0.0004 (95% CI, -0.0011 to 0.0019) mm/y (P<.001). All P values are vs placebo group. Overall, rosuvastatin was well tolerated with infrequent serious adverse cardiovascular events (6 participants [0.86%] had 8 events [1.1%] over 2 years).
Conclusions In middle-aged adults with an FRS of less than 10% and evidence of subclinical atherosclerosis, rosuvastatin resulted in statistically significant reductions in the rate of progression of maximum CIMT over 2 years vs placebo. Rosuvastatin did not induce disease regression. Larger, longer-term trials are needed to determine the clinical implications of these findings.
INTRODUCTION
Lipid-lowering therapy has been shown to reduce cardiovascular events in a large number of studies.1-3 A 19% reduction in coronary mortality has been recorded per 1.0-mmol/L (38.7 mg/dL) decrease in low-density lipoprotein cholesterol (LDL-C).4 Statins as well as other agents (bile acid sequestrants/niacin, probucol, antihypertensive medications, hypoglycemic agents) and lifestyle change also have been shown to slow the progression of and even regress atherosclerosis.5 This slowing of progression or induction of regression by statins has previously been demonstrated for coronary atherosclerosis as assessed by quantitative coronary angiography and intravascular ultrasound,5-7 and for carotid atherosclerosis as assessed via B-mode ultrasound measurement of carotid intima-media thickness (CIMT).5, 8-10 Most clinical trials of lipid-lowering efficacy have shown that lowering lipid levels is beneficial irrespective of baseline LDL-C level. However, the majority of such trials have been performed in high-risk populations, in individuals with high lipid concentrations, or in patients with existing cardiovascular disease.
Rosuvastatin is effective at lowering LDL-C concentrations.11-12 In addition, rosuvastatin, like other statins, has favorable effects on other components of the lipid and lipoprotein profile, such as raising high-density lipoprotein cholesterol (HDL-C) and reducing levels of total cholesterol and triglycerides.11, 13 The Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin (METEOR) study was designed to investigate the effect of a 40-mg dose of rosuvastatin on CIMT over 2 years in middle-aged individuals with low Framingham risk scores but with subclinical atherosclerosis.
RESULTS
Participant Enrollment and Characteristics
Individuals were enrolled from August 2002 to March 2004, and the last participant completed the study in May 2006. In total, 5751 individuals were screened and 984 were randomized. Individuals were initially screened for lipid concentrations. Those meeting lipid entry criteria were then assessed to exclude individuals who were not at low risk according to Framingham criteria. This eliminated the majority of individuals from the study. Individuals considered low risk and who met all other study entry criteria were sent for carotid ultrasound scans to determine eligibility by CIMT criteria. The modified intention-to-treat population consisted of 624 individuals in the rosuvastatin group (89%) and 252 in the placebo group (89%) (Figure 1). There were 172 participants in the rosuvastatin group (25%) who discontinued participation in the study and 74 in the placebo group (26%). Adverse events led to 79 withdrawals (11%) from the rosuvastatin group and 22 (8%) from the placebo group. Forty-seven individuals (6.7%) in the rosuvastatin group withdrew consent and 28 (9.9%) withdrew consent in the placebo group.
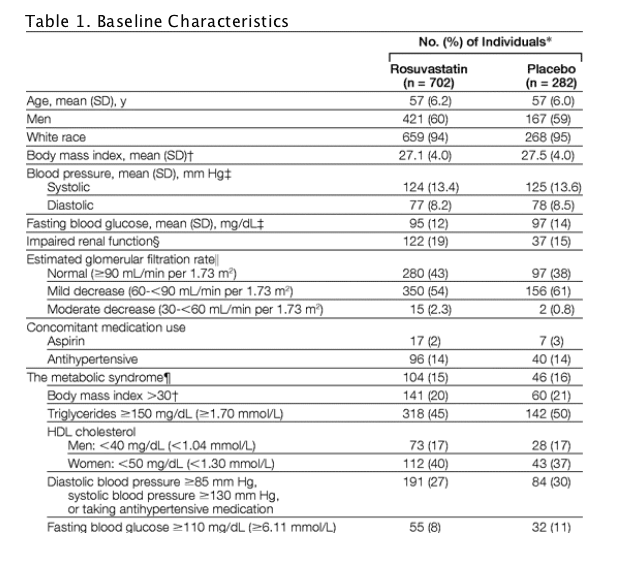
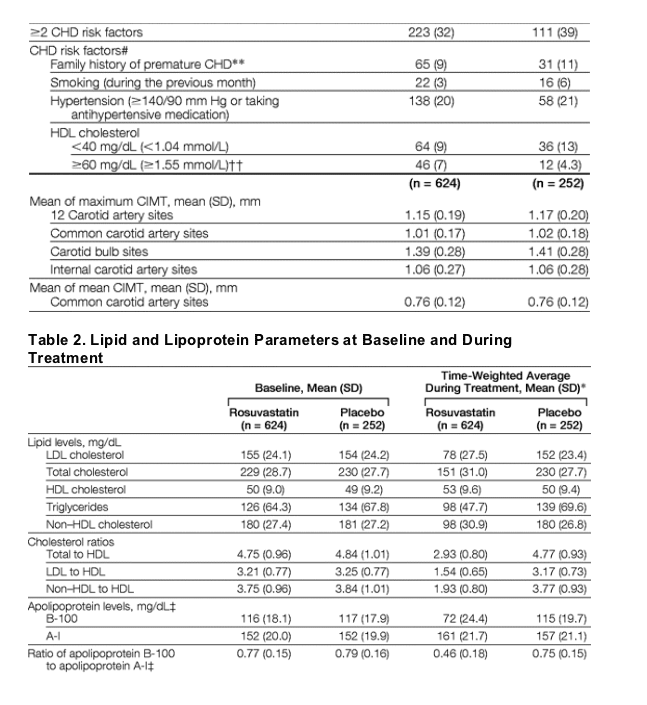
Baseline characteristics were well-balanced between the 2 groups (Table 1). In total, 967 participants (98% of the population) were at low Framingham risk, 16 participants (1.6%) were at moderate risk, and 2 individuals (1 enrolled with diabetes and 1 with undisclosed coronary artery disease, both representing deviations from the study protocol) were at high risk. Mean LDL-C level was 155 mg/dL (4.0 mmol/L) in the rosuvastatin group and 154 mg/dL (3.99 mmol/L) in the placebo group (Table 2). Baseline concentrations of total cholesterol, triglycerides, and HDL-C were similar between the 2 groups.
Effects of Rosuvastatin on Lipid and Lipoprotein Levels
Rosuvastatin treatment was associated with a 49% reduction in LDL-C level, a 34% reduction in total cholesterol level, an 8% increase in HDL-C level, and a 16% reduction in level of triglycerides (all P<.001 compared with placebo; Table 2).
Effects of Rosuvastatin on CIMT
In the rosuvastatin group, 624 participants had CIMT scans at 0 months, 618 participants at 6 months, 594 participants at 12 months, 554 participants at 18 months, and 538 participants at 24 months compared with 252, 252, 240, 222, and 211 participants, respectively, in the placebo group. All participants had duplicate scans at baseline. At 24 months, 514 participants in the rosuvastatin group and 202 participants in the placebo group had duplicate scans.
Compared with placebo, rosuvastatin significantly slowed progression of the maximum CIMT for the 12 carotid sites (rosuvastatin group: change in CIMT, -0.0014 mm/y [95% confidence interval {CI}, -0.0041 to 0.0014 mm/y]; placebo group: change in CIMT, 0.0131 mm/y [95% CI, 0.0087-0.0174 mm/y]; P<.001) (Table 3 and Figure 2). For all secondary CIMT end points (ie, segment-specific rates of change), similar results were observed (all P<.001, except for the change in internal carotid artery, which was P = .02) (Table 3).
Statistically significant regression was not observed in the rosuvastatin group (ie, the rate of change in the primary end point for the rosuvastatin group of -0.0014 mm/y [95% CI, -0.0041 to 0.0014 mm/y] was not significantly different from zero; P = .32) (Table 3). For secondary end points, significant regression was observed in maximum CIMT of the common carotid artery (-0.0038 mm/y [95% CI, -0.0064 to -0.0013 mm/y]; P = .004) but not for the other segments.
In the placebo group, as evidenced by the 95% CIs, there was significant progression for the primary and secondary CIMT end points. In contrast, significant progression was not observed in the rosuvastatin group for either the primary or secondary CIMT end points.
Subgroup Analyses
The effects of rosuvastatin in prespecified subgroups of individuals were evaluated. Individuals were divided according to their age, sex, geographical location, race, body mass index (calculated as weight in kilograms divided by height in meters squared), risk factors, presence of hypertension, and baseline mean values for each of the lipid and lipoprotein parameters. Results were robust for the rosuvastatin group and differences between the 2 groups were consistent across all prespecified subgroups. Although some differences in baseline characteristics existed between the US and European participants,26 there were no apparent differences in the baseline mean of maximum CIMT (1.15 mm in both the United States and Europe), and no apparent differences in treatment effects (differences in rates of change between the rosuvastatin and the placebo group were -0.0145 mm/y in Europe and -0.0135 mm/y in the United States).
Sensitivity Analysis
Several exploratory analyses were undertaken to assess the potential impact of missing data. Conservative analyses were performed to determine the possible effect of missing data due to early withdrawal. In a worst-case analysis, individuals in the placebo group had values imputed after withdrawal as if they had no further progression, while individuals in the rosuvastatin group were given imputed values after withdrawal as if they had progressed at the average rate of participants in the placebo group. The effect was to dilute the between-group difference in rate of change of the primary end point to -0.0117 mm/y (vs -0.0145 mm/y without imputation), which remained statistically significant (P<.001). Additional analyses were performed on a per protocol population and consistent results were obtained.
To assess the linearity of change in maximum CIMT between baseline and week 104, time-squared terms were included in the model in the intention-to-treat population. Neither term was statistically significant (P = .17 for the rosuvastatin group and P = .97 for the placebo group), indicating that the rate of change was constant over the 2-year period. Similar results were observed when this model was applied only to participants who completed the study.
Safety
The frequency of adverse events was similar between the treatment groups (Table 4). Myalgia was the most commonly occurring event (12.7% in the rosuvastatin group and 12.1% in the placebo group). Serious adverse cardiovascular events were infrequent (6 participants [0.86%] had 8 events [1.1%] in the rosuvastatin group vs 0% in the placebo group). There was 1 death during the study; a 64-year-old man in the rosuvastatin group developed paresthesias leading to amnesia, visual disturbances, and blindness over a 5-month period beginning 1 week prior to rosuvastatin exposure, and died 2 months after the last dose of study medication. The cause of death was reported to be Creutzfeldt-Jakob disease. The death was not considered related to treatment by the investigator.
Ischemic cardiovascular events were not specifically adjudicated in METEOR given the anticipated low frequency among the study population. However, 6 participants in the rosuvastatin group (0.86%) experienced treatment-emergent ischemic cardiovascular events during the 2 years of the study (3 experienced angina pectoris, 2 experienced acute coronary syndromes, and 1 participant with undisclosed coronary artery disease experienced a myocardial infarction). None of these events was considered to be related to treatment. No treatment-emergent ischemic cardiovascular events were reported in the placebo group. There were no cases of hepatitis, rhabdomyolysis (defined by the American College of Cardiology and the American Heart Association27 as muscle symptoms with creatine kinase elevation 10 times the upper limit of normal [ULN] and creatinine elevation usually with brown urine and urinary myoglobin), or renal failure.
Elevations in alanine aminotransferase higher than 3 times the ULN on 2 consecutive occasions were experienced by 4 individuals (0.6%) in the rosuvastatin group and 1 individual (0.4%) in the placebo group. Three participants (1 in the rosuvastatin group and 2 in the placebo group) experienced elevations of creatine kinase to higher than 10 times the ULN. The 1 participant in the rosuvastatin group who had experienced exercise-associated muscle pain with the creatine kinase elevation of higher than 10 times the ULN (3059 U/L) continued taking the study medication and creatine kinase levels subsequently declined to the normal range. Two individuals in the placebo group had creatine kinase values higher than 10 times the ULN (3700 U/L and 3724 U/L) after the qualifying visit but prior to receiving study medication. Neither individual had muscle symptoms and both completed the study with follow-up creatine kinase levels declining to the normal range. The frequency of proteinuria (a shift in dipstick urine protein from none or trace at baseline to 2+ during the study) was low (0.3% of participants in the rosuvastatin group and 0.4% of participants in the placebo group at the final visit). There was a decrease in glomerular filtration rate for both treatment groups over the 2 years; mean absolute change from baseline to final visit was -3.82 mL/min per 1.73 m2 for the rosuvastatin group and -4.47 mL/min per 1.73 m2 for the placebo group. Overall, the frequency of adverse renal effects was low and similar in both groups.
COMMENT
Results of the METEOR trial show that a 40-mg dose of rosuvastatin significantly slowed progression of atherosclerosis both overall and for individual carotid segments as assessed by CIMT measurements. Furthermore, rosuvastatin lowered LDL-C concentration by 48.8% and raised HDL-C concentration by 8.0% compared with baseline concentrations; these lipid effects of rosuvastatin are consistent with previous studies.6, 11-12,28 The 40-mg dose of rosuvastatin used in this study is not a recommended starting dose but was chosen to provide the maximum efficacy expected to slow or delay progression of atherosclerosis or even facilitate regression.
The findings of the METEOR trial compare well with results from other trials on the effects of statin therapy on progression of CIMT.5, 8 Nearly all of the previously published lipid-lowering trials showed a reduced progression in the treatment group compared with the control (placebo) groups.29 However, most of these studies were performed in secondary prevention populations or in high-risk patients with elevated LDL-C levels. The METEOR trial expands the findings of previous trials to asymptomatic patients, who have no current requirement for statin use, and have low Framingham risk scores, evidence of subclinical carotid atherosclerosis, and modestly elevated lipid concentrations.
Rosuvastatin did not result in statistically significant regression of CIMT. Given the intima-media thickness inclusion criteria, the participants did not have advanced atherosclerosis and so it may have been optimistic to expect rosuvastatin to cause regression. We chose low-risk individuals with only mild atherosclerosis for the study to justify the use of a placebo group. In contrast to the significant progression of atherosclerosis in the placebo group, no significant progression was observed in the rosuvastatin group. These results indicate that even in this low-risk, middle-aged population with mild atherosclerosis, cholesterol management with rosuvastatin slows the progression of CIMT compared with placebo over a 2-year period. This suggests that rosuvastatin can affect even small increases of CIMT associated with early abnormalities of arterial (presumably intimal) structure.
In comparison, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID),6 which included patients with more advanced atherosclerosis, treatment with a 40-mg dose of rosuvastatin for 2 years resulted in regression of coronary atherosclerosis compared with baseline, as assessed by intravascular ultrasound. Presumably, the presence of more advanced disease in ASTEROID allowed detection of regression that was not observed in METEOR. Although a reduction in clinical events by rosuvastatin has not yet been demonstrated, increased CIMT has been linked to increased cardiovascular risk in asymptomatic adults30-32 and a reduced progression of CIMT in statin trials is congruent with a reduction in cardiovascular events.33
Several issues regarding the design and findings of the study need to be addressed. First, progression of maximum CIMT over the 12 sites, including the common carotid, carotid bulb, and internal carotid, was chosen as the primary end point because it best reflects changes in atherosclerosis.34 This is in contrast to several other trials in which the progression of the common CIMT was used as the primary end point, based on the rationale that a change in mean common CIMT predicts cardiovascular disease.35 However, in the present study, rosuvastatin slowed progression of mean common CIMT in a similar magnitude to that of the maximum CIMT.
Second, the number of individuals randomized was more than intended based on the protocol. This was due to the difficulty of stopping recruitment while potentially eligible participants in the screening period were awaiting central reading of eligibility CIMT scans.
Third, the modified intention-to-treat analysis was based on a smaller number of participants than was randomized. This was due to exclusion of individuals who withdrew within 6 months of randomization and did not have a follow-up ultrasound scan to determine the change in CIMT. To examine whether withdrawal (ie, absence of a postbaseline CIMT measurement) might have affected our results, we studied whether baseline characteristics, risk factors, and assignment of treatment were related to CIMT measurement during the study. In these analyses (data available on request), neither baseline characteristics, risk factors, nor treatment assignment were related to absence of a postbaseline CIMT measurement. These results suggest that any bias in the study findings that might result from the exclusion of individuals with no postbaseline measurement was minimal.
METEOR is a study of CIMT progression using 2 core laboratories (1 in the United States and 1 in Europe). Although we used an identical ultrasound protocol, rigorous quality control, and identical reading stations for offline assessment of CIMT, some differences in interpretation might have remained. However, the treatment effects in Europe were similar to those in the United States. Finally, a unique feature of METEOR was that potential drift in CIMT measurements was excluded by having all scans of the same individual read in random order by 1 reader over a short period at the end of the study.
A 40-mg dose of rosuvastatin was well tolerated during the 2-year study period and showed a similar safety profile to that of placebo. The majority of adverse events were mild to moderate in intensity. The most frequently reported adverse event was myalgia.
In conclusion, the findings of METEOR demonstrate that in middle-aged adults with Framingham risk scores lower than 10% and evidence of subclinical atherosclerosis, rosuvastatin treatment resulted in statistically significant reductions in the rate of progression of maximum CIMT during a 2-year period compared with placebo. Rosuvastatin did not induce regression overall. Larger, longer-duration randomized trials focused on clinical events are needed to determine the practice implications of these findings.
METHODS
METEOR was a 2-year, double-blind, placebo-controlled trial comparing a 40-mg dose of rosuvastatin with placebo in middle-aged asymptomatic individuals with moderately elevated cholesterol and low risk of cardiovascular disease according to the National Cholesterol Education Program Adult Treatment Panel III guidelines criteria (0-1 risk factor or 2 risk factors with a 10-year coronary heart disease risk <10%)14 as published previously.15 All participants were provided counseling regarding therapeutic lifestyle changes. Participants had evidence of thickening of the walls of the extracranial carotid arteries as measured by B-mode ultrasound. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonisation of Good Clinical Practice guidelines, and appropriate regulatory requirements. The study protocol also was approved by the appropriate institutional review board or independent ethics committee at each site. All participants provided written informed consent.
Main inclusion criteria were age 45 to 70 years (men) or 55 to 70 years (women); screening LDL-C level of 120 to less than 190 mg/dL (3.1-<4.9 mmol/L) for those with only age as a coronary heart disease risk factor or 120 to less than 160 mg/dL (3.1-<4.1 mmol/L) for individuals with 2 or more coronary heart disease risk factors and a 10-year risk of coronary heart disease events of less than 10%14; HDL-C level of 60 mg/dL or lower (1.6 mmol/L); level of triglycerides lower than 500 mg/dL (<5.7 mmol/L); and maximum CIMT measurements between 1.2 mm and less than 3.5 mm from 2 separate ultrasound examinations. This lower boundary for CIMT measurement actually identifies a relatively high wall thickness in the general population. Thus, these participants were clinically at low risk, although their intima-media thickness criteria indicated the presence of subclinical atherosclerosis. This disconnect made it difficult to recruit study participants. Exclusion criteria included use of lipid-lowering therapies in the previous 12 months, clinical evidence of coronary artery disease or other peripheral atherosclerotic disease, prior revascularization procedures, 10-year coronary heart disease risk 10% or more, diabetes mellitus, uncontrolled hypertension or familial hypercholesterolemia, or serum creatinine concentration higher than 2 mg/dL (>177 μmol/L). Race/ethnicity was self-reported by the individuals and recorded by the investigator or study coordinator.
Carotid ultrasound examinations were performed twice before randomization, once each at 6, 12, and 18 months after randomization, and then twice at the end of 24 months of study treatment. Adverse events were reported every 3 months at clinic visits or at interim periods when notified.
Eligible individuals were randomized to either the placebo or rosuvastatin group in blocks of 7 (5 to the rosuvastatin group and 2 to the placebo group) at each clinical site. Blinded study medication was supplied in individual numbered bottles prepared prior to the clinic visits and eligible individuals were allocated study medication sequentially. Investigators checked adherence but were unaware of treatment allocations for the duration of the study.
End Points
The primary end point was annualized rate of change in maximum CIMT based on all scans performed during the 2-year study period from each of the 12 carotid artery sites (near and far walls of the right and left common carotid artery, carotid bulb, and internal carotid artery). Secondary CIMT end points were annualized rate of change in maximum CIMT derived from the near and far walls of: the right and left common carotid artery; the right and left carotid bulb; the right and left internal carotid artery; and annualized rate of change in mean CIMT for the near and far walls of the right and left common carotid artery.
Sample size in the rosuvastatin group was estimated to demonstrate statistically significant regression at 2 years, assuming a 2-sided level of statistical significance of .05, 80% power, and a mean (SD) decrease in CIMT of -0.008 (0.058) mm/y.16-20 This resulted in a requirement of 415 individuals for the rosuvastatin group. Sample size in the placebo group (n = 167) was then determined to provide more than 90% power for the between-group comparison, assuming a mean (SD) increase in CIMT in the placebo group of 0.012 (0.058) mm/y.16-19,21 Therefore, participants were randomized in a 5:2 ratio to have sufficient power for both objectives. Allowing for a 30% withdrawal rate over the 2 years of the study, the intention was to enroll 840 participants randomized at a ratio of 5 participants to the rosuvastatin group and 2 participants to the placebo group.
B-mode Ultrasound Method
Standardized longitudinal B-mode images were obtained of the near and far walls of the 3 segments of the carotid artery, as detailed elsewhere.15 The common carotid artery was assessed in the segment extending from 10 to 20 mm proximal to the tip of the flow divider. The carotid bifurcation was assessed from the tip of the flow divider, extending 10 mm proximal to the tip of the flow divider. The internal carotid artery was assessed in the 10 mm distal to the tip of the flow divider. The Meijer carotid arc was used to image the artery at prespecified angles. All ultrasound scans were read with Image Pro software (Microsoft, Redmond, Wash), using a uniform reading protocol that ensured standardized settings across reading stations and core laboratories. The image boundaries were marked manually. For CIMT measurements, trailing edges were traced on the near wall boundaries and leading edges on the far wall boundaries. Measurements were performed on images from selected angles. For the right carotid artery, measurements were performed with the Meijer carotid arc at 60, 90, 120, 150, and 180 and for the left carotid artery at 300, 270, 240, 210, and 180. For the near and far walls of the right and left carotid bifurcation and internal carotid artery, measurements were made only of the maximum CIMT at all selected angles. For the common carotid artery, measurements were made of both the mean and maximum CIMT of each wall at all selected angles.
All readers completed a uniform training program. All 7 scans were read in a batch fashion and in random order after each individual had finished the study to exclude any potential for reader drift in measurements. Batches were read by a single reader to avoid interreader variability.
The intraclass correlation coefficient for the mean of the 12 site-specific maximum CIMT values was 0.93 based on the 2 duplicate scans at baseline (n = 876 paired values); the mean (SD) absolute difference was 0.056 (0.049) mm, the median absolute difference was 0.042 (range, 0 to 0.320) mm, and the mean (SD) difference was 0.002 (0.074) mm. Duplicate scans at the end of study (n = 716 paired values) yielded almost identical results.
Laboratory Parameters
All laboratory services were performed by Covance Central Laboratory Services Inc (Indianapolis, Ind, and Geneva, Switzerland).
Fasting lipid and lipoprotein levels were measured at baseline, 3 months, 1 year, and 2 years. Apolipoprotein levels were measured at baseline and at the end of the study. The central laboratories were certified for standardization of lipid analysis as specified by the Standardization Program of the US Centers for Disease Control and Prevention and the National Heart, Lung, and Blood Institute Part III Lipid Standardization Program.22
Clinical laboratory assessments included liver function tests, creatine kinase level, creatinine level, electrolytes, fasting serum glucose, hematological measurements, and urinalysis, which were measured every 3 months. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease glomerular filtration rate formula.23
Safety Assessments
Safety was assessed by vital signs, adverse event reports, clinical laboratory data, and electrocardiograms. Blood pressure and resting heart rate were measured at every clinic visit during the study. Adverse events were reported at each study visit by means of standard questions. Clinically significant abnormal laboratory values, vital signs, or other physical findings were recorded as adverse events. Electrocardiograms were performed at baseline and at the end of the study.
Data Analysis
Data on CIMT were analyzed according to a modified intention-to-treat principle in all individuals with at least 1 additional CIMT reading after the baseline studies.
A multilevel, repeated measures, linear mixed-effects model was used for the analysis of the primary and secondary CIMT end points.17, 24-25 The levels used for the data were defined by (1) the individual participant and (2) the carotid artery site within the participant. The repeated measure was time. The model was specified in terms of fixed effects for carotid artery site, age, sex, scan reader, ultrasound machine, treatment group, time, and the interaction between treatment group and time. Time as a continuous variable was the interval from the date of randomization to date of CIMT measurement. To assess the linearity of changes in CIMT values across the study measurements, time-squared terms were included in the model. Random effects within the model were intercept and slope for individual participants and sites within participants.
The dependent variable was maximum CIMT. For analysis of the primary end point, each participant provided up to 12 maximum CIMT values at each assessment (ie, 1 for each carotid site that could be visualized). For secondary end points, each participant provided up to 4 CIMT values (the near and far walls of the right and left common carotid artery, internal carotid artery, or carotid bulb) at each assessment. The model used the rate of change at each site for each participant to create estimates of annualized rate of change for the end point in each treatment group. Site-specific CIMT values within participants were likely to be correlated. The model allowed for this by nesting site effects within participant effects, reflecting the multilevel nature of the data. For tests of statistical significance, variability was assessed within treatment groups with the participant being the unit of observation.
For analysis of the primary end point, a sequential testing procedure was used. First, the difference in annualized rate of change between the 2 randomized treatment groups was tested by evaluating the statistical significance of the time x treatment interaction term. If this was statistically significant (P<.05), and the annualized rate of change within the rosuvastatin group was negative, then a further test for significant regression was performed to compare change after treatment with rosuvastatin with no change. Sequential testing also was performed for secondary end points. Nominal P values are presented throughout without adjustment.
The model fitted regression lines to profiles of CIMT values consisting of 2 values prior to randomization, 3 values from visits during the treatment period, and values from 2 end of study visits. Imputation methods were not necessary because if a participant withdrew from the study with an incomplete profile after the ultrasound visit at 6 months, the annualized rate of change for that individual could still be estimated from the data available. This regression approach was appropriate because CIMT values (and therefore the primary and secondary end points based on them) were expected to change in a linear fashion over time.
To deal with missing data, regression lines were fitted using restricted maximum likelihood methods to site-specific CIMT values rather than to means over carotid sites. The reason for this is that some carotid artery sites were consistently more difficult to visualize than others, giving rise to missing data for approximately 1% to 5% of scans, depending on the site. As sites also differed in thickness, simple averaging of CIMT values over 12 carotid sites would be biased, and regression lines fit to such data could have exhibited additional variability. Therefore, the model-based approach described above was used with the essential feature being the estimation of rates of change from regression lines for the 2 randomized groups from the model.
Percentage change from baseline in lipid and lipoprotein levels was assessed using analysis of covariance with terms for treatment and region in the model. Missing data at the end of the study were accounted for using last observation carried forward. Time-weighted averages also were calculated for lipid variables. These more precisely reflect change during the 2-year treatment period and were defined as the lipid value multiplied by the number of days since the last lipid assessment, summed for all lipid observations and divided by the sum of days between all visits.
The safety population consisted of all participants who received at least 1 dose of study medication during the 104-week treatment period. Safety data were summarized by actual treatment received. Although the study was not powered to make safety comparisons, and formal tests of statistical significance were not performed, there was a 50% chance of observing at least 1 case of any adverse event among 700 participants treated with rosuvastatin with a true incidence of 1 episode per 1000 patients.
S-Plus version 6.2 (S-PLUS, Insightful Corp, Seattle, Wash) was used to analyze CIMT data and SAS version 8.2 (SAS Institute Inc, Cary, NC) was used for analyses of other data. All statistical tests were 2-sided and P values less than .05 were considered significant.
|
|
| |
| |
|
|
|