| |
rHGH Reduced Belly Fat; Safety Concerns: glucose, limb fat
|
| |
| |
"Recombinant Human Growth Hormone to Treat HIV-Associated Adipose Redistribution Syndrome: 12-Week Induction and 24-Week Maintenance Therapy"
JAIDS Journal of Acquired Immune Deficiency Syndromes:Volume 45(3)1 July 2007pp 286-297
Grunfeld, Carl MD, PhD*; Thompson, Melanie MD; Brown, Stephen J MD; Richmond, Gary MD; Lee, Daniel MD; Muurahainen, Norma MD, PhD; Kotler, Donald P MD#; on behalf of the Study 24380 Investigators Group
From the *Division of Endocrinology, University of California, San Francisco Veterans Affairs Medical Center, San Francisco, CA; AIDS Research Consortium, Atlanta, GA; AIDS Research Alliance, West Hollywood, CA; Richmond Clinic, Fort Lauderdale, FL; University of California, San Diego, Antiviral Research Center, San Diego, CA; EMD Serono Inc., Clinical Development, Rockland, MA; and #Gastrointestinal Immunology, Columbia/St. Luke's-Roosevelt Hospital, New York, NY.
Received for publication September 29, 2006; accepted February 12, 2007.
Funding for this study was provided by EMD Serono Inc., Rockland, MA.
Presented in part at the 16th Annual AIDS Conference, Toronto, Ontario, Canada, August 13-18, 2006.
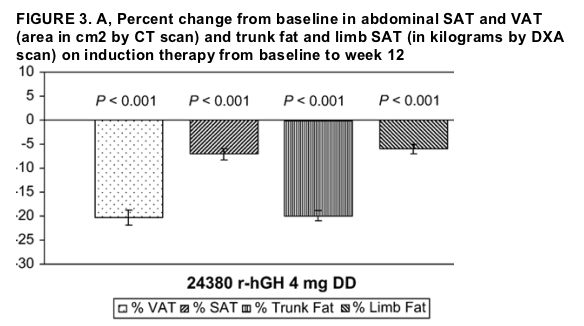
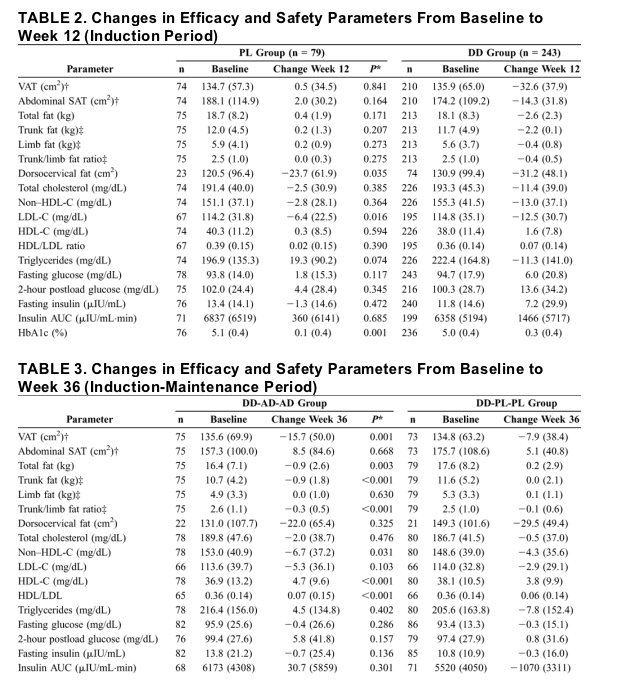
"....in terms of percentage reductions in each of the body composition parameters from baseline to week 12 (Fig. 3), the DD group (patients receiving rHGH) had a mean 20.3% reduction in VAT area during weeks 1-12, although the PL group showed no decrease (3.6%; P < 0.001). The DD group also had a 20% reduction in trunk fat, 7.1% reduction in abdominal SAT, and 6.0% reduction in limb fat. In contrast, the PL group showed gains in trunk fat, abdominal SAT, and limb fat (1.4%, 1.6%, and 2.8% respectively; P < 0.001 for each between-group difference).... At 36 weeks, the DD-AD-AD and DD-PL-PL groups still showed some reduction from baseline in mean VAT (-15.7 cm2 and -7.9 cm2, respectively), but the between-group difference was not statistically significant (see Table 3). In contrast, after 36 weeks, neither group showed a net loss from baseline of abdominal SAT (8.5 cm2 and 5.1 cm2, respectively) or limb fat (0.0 kg and 0.1 kg, respectively). (QUESTION: did the not showing loss of SAT or limb fat after 36 weeks result because of patient drop outs after 12 weeks, during which time SAT or limb fat loss occurred?)...
.... In the DD group, from baseline to week 12, fasting glucose levels (see Table 2) rose by 7.9% and 120-minute glucose levels rose by 19.2% (P < 0.001 for both) compared with 3.1% and 6.8%, respectively, in the PL group (P ≦ 0.017). Fasting serum insulin and insulin area under the curve (AUC) also rose more in the DD group than in the PL group during the induction period, with increases in the DD group of 133.5% for insulin and 79.5% for insulin AUC compared with 42.1% and 27.5% for these variables in the PL group (P < 0.05 for between-group comparisons). During this period, the mean change in HbA1c was also significantly greater (P < 0.001) in the DD group than in the PL group (0.3%, an increase of 6.5%, vs. 0.1%, an increase of 2.7%). At week 12, however, all patients in both groups had an HbA1c level less than 7%, the highest desirable level in managing diabetes.14 In the DD group at week 12, mean fasting glucose (100.7 mg/dL) remained close to normal (100 mg/dL) and mean 2-hour postload glucose (113.8 mg/dL) was normal (<140 mg/dL) (see Table 3).....By week 36, mean fasting glucose, insulin, and insulin AUC returned to baseline levels (see Table 3; Fig. 4) in both groups that had been given r-hGH induction (DD-AD-AD and DD-PL-PL groups). (QUESTION: same question, were improvements after 12 weeks in glucose due to patient drop outs??)
Abstract
Background: HIV-associated adipose redistribution syndrome (HARS) is an HIV-associated disorder characterized by excess truncal fat, including visceral adipose tissue (VAT).
Methods: From baseline to week 12 in this randomized, double-blind, placebo (PL)-controlled, multicenter trial investigating effects of recombinant human growth hormone (r-hGH; Serostim; EMD Serono Inc., Rockland, MA) in patients with HARS, 325 received induction (4 mg/d of r-hGH) or PL. At week 12, patients who initially received induction were rerandomized to 2 mg of r-hGH on alternate days (maintenance) or PL to week 36. Patients who initially received PL later received 4 mg/d of r-hGH. Change in VAT was the primary outcome. Key secondary outcomes included changes in non-high-density lipoprotein cholesterol (non-HDL-C) and limb fat.
Results:
At week 12, induction therapy resulted in decreased VAT (-32.6 vs. 0.5 cm2; P < 0.001), limb fat (-0.4 vs. 0.2 kg; P < 0.001), and non-HDL-C (-13.0 vs. -2.8 mg/dL; P = 0.023) compared with PL. On r-hGH induction-maintenance (baseline to week 36), patients sustained losses in VAT and trunk fat but not losses of subcutaneous fat in the abdomen or limbs. Also, non-HDL-C remained significantly decreased on r-hGH but not on PL maintenance.
Conclusions: In patients with HARS, r-hGH induction-maintenance therapy produces greater relative losses of VAT and trunk fat than of subcutaneous fat and also has beneficial effects on the lipid profile.
Safety
Changes in Insulin and Glucose Metabolism: Baseline to Week 12
In the DD group, from baseline to week 12, fasting glucose levels (see Table 2) rose by 7.9% and 120-minute glucose levels rose by 19.2% (P < 0.001 for both) compared with 3.1% and 6.8%, respectively, in the PL group (P ≦ 0.017). Fasting serum insulin and insulin area under the curve (AUC) also rose more in the DD group than in the PL group during the induction period, with increases in the DD group of 133.5% for insulin and 79.5% for insulin AUC compared with 42.1% and 27.5% for these variables in the PL group (P < 0.05 for between-group comparisons). During this period, the mean change in HbA1c was also significantly greater (P < 0.001) in the DD group than in the PL group (0.3%, an increase of 6.5%, vs. 0.1%, an increase of 2.7%). At week 12, however, all patients in both groups had an HbA1c level less than 7%, the highest desirable level in managing diabetes.14 In the DD group at week 12, mean fasting glucose (100.7 mg/dL) remained close to normal (100 mg/dL) and mean 2-hour postload glucose (113.8 mg/dL) was normal (<140 mg/dL) (see Table 3). (QUESTION: were there patient dropouts??, Did improved glucose measures result because patients with abnormalities drop out??)
Changes in Insulin and Glucose Metabolism: Weeks 12 to 36 and Baseline to Week 36
By week 36, mean fasting glucose, insulin, and insulin AUC returned to baseline levels (see Table 3; Fig. 4) in both groups that had been given r-hGH induction (DD-AD-AD and DD-PL-PL groups). There was a trend toward an increase in percent change in 2-hour postload glucose from baseline to week 36 in the DD-AD-AD group (11.1%; P = 0.052; not shown), but the absolute change (5.8 mg/dL) was not significant; there was no significant change from baseline to week 36 in the DD-PL-PL group. Mean concentrations of fasting glucose, 2-hour postload glucose, and HbA1c at week 36 were within the normal range in the DD-AD-AD and DD-PL-PL groups (see Table 3). In addition, all but 1 patient in the DD-AD-AD and DD-PL-PL groups had HbA1c levels less than 7% at week 36. No subject required glucose-lowering agents.
Adverse Event Profile: Baseline to Week 12
More than 95% of AEs in each group were mild or moderate in severity. The percentage of patients reporting any AE from baseline to week 12 was high in both groups but higher in the DD group (93.9%) than in the PL group (79%) (P < 0.001). The AEs associated with r-hGH were similar to those observed on r-hGH treatment in previous trials of adults with growth hormone (GH) deficiency,17 HIV-associated wasting,18 and HARS.5 From baseline to week 12, there were significantly higher rates of peripheral edema (46.3% vs. 4.9%) and arthralgia (38.9% vs. 17.3%) on r-hGH than on PL (both P < 0.001). A somewhat larger percentage of subjects on r-hGH than on PL (17.2% vs. 8.6%; P = 0.073) reported AEs of severe-grade toxicity (defined per toxicity guidelines as significant impairment of functioning).
There were no patient deaths in either group during induction. Five patients experienced a total of 6 SAEs (events requiring hospitalization or procedures), including abnormal liver function test results, hypokalemia, migraine, myocardial ischemia, depression, and abdominal pain. There was no significant between-group difference in the prevalence of SAEs; 2 were reported in the PL group (2.5%) and 4 in the DD group (1.6%). Only the migraine was rated as possibly related to study drug. During induction, 34 patients in the DD group (13.9%) and 2 patients in the PL group (2.5%) discontinued treatment because of AEs (P = 0.004).
Adverse Event Profile: Weeks 12 to 36
There were no significant differences in the prevalence of AEs, SAEs, or study discontinuations between treatment groups during the maintenance period (weeks 12-36). AEs were reported by 74% of subjects in the DD-AD-AD group and by 68% in the DD-PL-PL group. The only difference in prevalence of AEs between groups was for upper respiratory tract infections (16.1% in the DD-PL-PL group and 7.6% in the DD-AD-AD group). SAEs were reported by 7.6% in the DD-AD-AD group and by 5.4% in the DD-PL-PL group during the maintenance period. Withdrawals or discontinuations for any reason during maintenance were similar in the DD-AD-AD (17%) and DD-PL-PL (16%) groups.
Rates of discontinuation from the study for SAEs were also similar (6.5% in the DD-AD-AD group and 7.5% in the DD-PL-PL group). Six SAEs were reported in 3 patients in the DD-PL-PL group, including cardiorespiratory arrest resulting in death in 1 patient, pulmonary embolism with phlebitis in 1 patient, and pyelonephritis with diarrhea and Clostridium difficile sepsis in 1 patient. Four SAEs occurred in 2 patients in the DD-AD-AD group: pneumonia in 1 patient and diarrhea, pancreatic insufficiency, and nephrolithiasis in 1 patient. In the PL-PL-DD group, 3 SAEs (groin abscess, pneumonia, and cutaneous basal cell carcinoma) were reported in 3 patients. Only the skin cancer was considered possibly related to study drug. Between-group differences were not statistically significant. A patient in the PL-PL-DD group was diagnosed with non-small cell adenocarcinoma of the lung 111 days after study onset while receiving PL, leading to study termination; the patient died 89 days after diagnosis.
As many as 40% of adults with HIV infection develop excessive accumulation of adipose tissue in the trunk, including accumulation of visceral (deep abdominal) adipose tissue (VAT), which may or may not be accompanied by loss of subcutaneous adipose tissue (SAT) from the abdomen, face, limbs, or buttocks; fat accumulation in the upper back or neck (dorsocervical region); or both.1,2 It has been proposed that such patients manifest HIV-associated adipose redistribution syndrome (HARS).3-5 Patients with HARS, like those with HIV-related lipoatrophy without excess VAT,1,2 often manifest glucose and lipid abnormalities and may be at greater risk for developing abnormalities of glucose and lipid metabolism and cardiovascular disease (CVD) than HIV-infected patients without fat maldistribution.6-8 HIV-infected patients with fat distribution abnormalities may also experience uncomfortable physical symptoms8 and have poor body image and impaired quality of life.9-11 At present, no drugs are approved for the treatment of HARS.
In a previous double-blind, randomized, placebo (PL)-controlled trial (study 22388) of patients with HARS without diabetes, recombinant human growth hormone (r-hGH) at a dosage of 4 mg/d for 12 weeks significantly reduced VAT and trunk fat, although improving lipid metabolism, body image, and quality of life parameters, as compared with PL.5,12 Treatment was accompanied by mild to moderate side effects, but few individuals experienced serious adverse events (SAEs). Study 22388 also showed that r-hGH dosed at 4 mg on alternate days (AD) maintained the effects of r-hGH on VAT and other parameters for another 12 weeks.5,12
The present clinical trial (study 24380) was conducted to confirm the impact of r-hGH dosed at 4 mg/d for 12 weeks in HARS in a larger sample of patients and to investigate the impact of maintenance therapy based on a lower dose of r-hGH (2 mg on AD) for 24 weeks.
METHODS
Study Design
As shown in Figure 1, for the initial 12 weeks of treatment, eligible patients were randomized in a 3:1 ratio to receive induction therapy, 4 mg of r-hGH dosed daily (group A, DD) or PL (group B, PL). At week 12, subjects in group A were rerandomized in a 1:1 ratio to receive maintenance therapy, 2 mg of r-hGH on AD or PL on AD, from weeks 12 through 36. Subjects from group A, on r-hGH induction and r-hGH maintenance, are referred to as the DD-AD-AD group; those from group A on induction therapy with r-hGH followed by PL maintenance are the DD-PL-PL group. Subjects in group B, who received PL from baseline to week 24 and then received 4 mg/d of r-hGH for weeks 24 through 36, are the PL-PL-DD group.
The prespecified primary efficacy parameter was the absolute change from baseline to week 12 in area of VAT on a cross-sectional computed tomography (CT) scan at L4-L5. Secondary efficacy endpoints included changes in other body composition parameters, serum lipids, body image, and quality-of-life variables (results for body image and quality of life are reported separately).13
Safety data included glucose and insulin parameters, viral load, CD4 cell counts, adverse events (AEs), insulin-like growth factor (IGF)-I and its main binding protein (IGF-BP3), hemoglobin A1c (HbA1c; an indicator of adequacy of glucose control over a 3-month period),14 and standard hematologic and clinical chemistry parameters.
The primary prespecified criterion of efficacy for maintenance therapy at week 36 was maintenance failure rate, defined as the proportion of subjects originally given 4 mg/d of r-hGH 4 for weeks 1 through 12 who succeeded in losing VAT during baseline to week 12 but then regained more than 50% of their VAT loss by week 36 (see statistical analysis). Failure rates were also compared between those assigned to PL or 2 mg of r-hGH on AD for weeks 12 through 36. Mean changes from baseline to week 36 in VAT, other body composition parameters, lipid profile, and safety parameters were also examined.
Randomization was stratified by gender and implemented by a central system operated by an independent vendor (Clinphone, Nottingham, United Kingdom), assigning patients to treatment using a blinded computer-generated randomization list. The trial was conducted according to the Declaration of Helsinki principles and Good Clinical Practice. Independent institutional review boards approved the protocol at each site. Written informed consent was obtained from each patient before screening.
Study Subjects
Eligibility criteria were the same as for the previous trial.5 Patients were between 18 and 60 years old, had documented HIV infection, had been on stable antiretroviral therapy (ARVT) for ≥30 days, and agreed to continue on ARVT while on study. They also had fasting glucose <110 mg/dL, 2-hour postload glucose on oral glucose tolerance testing (OGTT) results <140 mg/dL, and evidence of excess abdominal adipose tissue as determined by waist/hip ratio (WHR) ≥0.95 and waist circumference (WC) >88.2 cm for men and WHR ≥0.90 and WC >75.3 cm for women.5 The WHR criteria are similar to those identified as indicative of abdominal adiposity in the literature on obesity and are known to be associated with increased cardiovascular risk.15,16
Patients were excluded if they had active systemic infection, unstable or untreated hypertension (≥140/90 mm Hg), acute illness treated in an intensive care unit, a history of pancreatitis, carpal tunnel syndrome (unless resolved by surgical release), diabetes mellitus, malignancy (except for limited cutaneous Kaposi sarcoma or excised basal cell or squamous cell skin carcinoma), angina pectoris, coronary artery disease, or any disorder associated with moderate to severe edema. Patients must not have been receiving insulin or insulin-sensitizing agents, systemic glucocorticoids, or weight reduction agents for 3 months before screening or therapy for HIV-associated wasting (eg, anabolic steroids other than testosterone replacement, appetite stimulants, r-hGH) for 12 months before screening. Lipid-lowering agents were permitted if they were started at least 8 weeks before study entry.
Treatment and Assessment
The 4-mg induction dose of r-hGH (Serostim; EMD Serono, Rockland, MA) and its PL were given as 1.0-mL single subcutaneous injections each evening. The 2-mg AD maintenance dose of r-hGH and its PL were given as single 0.5-mL subcutaneous injections every other evening. Active and PL study drug were labeled and packaged identically, and doses were sequentially numbered. Patients were taught to self-inject according to the prescribed sequence. The protocol for dose adjustments for weight and toxicity was the same as used previously.5
Study visits were scheduled at screening; baseline; and weeks 2, 4, 12, 16, 24, 26, 28, and 36. CT and dual x-ray absorptiometry (DXA) scans to assess fat distribution were obtained at baseline, week 12, and week 36. OGTT and lipid profiles were obtained after a minimum 12-hour fast. These and serum IGF-I, IGF-BP3, HIV-1 RNA, and testosterone levels and CD4 T-cell count were obtained at baseline, week 12, week 24, and week 36. HbA1c levels were assessed at baseline and weeks 4, 12, and 36 by affinity chromatography. At each visit, standard hematologic and biochemistry panels, physical examinations, and reporting of AEs using the Medical Dictionary for Regulatory Activities (MedDRA), version 8.0 (MSSO, Reston, VA) were conducted. Laboratory testing was performed centrally (Esoterix Laboratories, Calabasas Hills, CA).
Statistical Analysis
Data were analyzed in the modified intention-to-treat (ITT) population, which included subjects who received at least 1 dose of study drug and who had follow-up data. There were separate analysis plans for the initial 12-week induction treatment period and the 24-week maintenance period. The week 12 analysis was the primary analysis. The primary efficacy parameter, change from baseline to week 12 in absolute area of VAT, was analyzed using a nonparametric ANCOVA model with effects for treatment and gender, with baseline VAT as a covariate. The major efficacy endpoint for the maintenance phase of the study was the percentage of patients regaining >50% of the VAT they had lost during induction (weeks 1-12). Maintenance therapy was considered efficacious if, during maintenance (weeks 12-36), no more than half of the subjects who had lost VAT regained >50% of the amount they lost. Mean changes in VAT, other body composition parameters, and lipid parameters were examined in the ITT sample from baseline to week 36 and from weeks 12 to 36 as well. The trial was not powered statistically to test differences in changes in study endpoints between maintenance groups, however.
Between-group differences in continuous secondary efficacy parameters were analyzed using raw data with an ANOVA model, including effects for treatment, gender, and treatment-by-gender interaction, when parametric model assumptions were met or, using ranked data, when parametric assumptions were not met. Within-group differences were analyzed using the Wilcoxon signed rank test. Safety results were summarized for the population of patients who received at least 1 dose of study drug and had follow-up data (n = 325 for induction, n = 258 for maintenance). Between-group differences in categoric variables were analyzed using the Fisher exact test.
RESULTS
Study Subjects
Between May and November 2004, 537 patients consented to participate and 326 (61%) were randomized (Fig. 2); 211 patients failed to meet entry criteria. Most who failed screening did so because of the presence of glucose abnormalities (fasting serum glucose >110 mg/dL or 2-hour postload glucose >140 mg/dL [22.2% of patients screened]).
The modified ITT population for induction therapy (weeks 1-12) included 322 patients who received at least 1 injection of study drug and had at least 1 postbaseline efficacy assessment during induction (79 in the PL group and 243 in the DD group). The safety population for this period included 325 patients who received at least 1 injection of study drug and had follow-up safety data during induction (81 in the PL group and 244 in the DD group). Two hundred and 74 completed the week 12 visit (200 in the DD group and 74 in the PL group).
Of the 200 patients in the DD group who completed the week 12 visit, 185 were randomly allocated to maintenance therapy with r-hGH or PL and received study drug (maintenance period safety population). Of the 76 who initially completed the PL group, 73 received an additional 12 weeks of PL followed by 12 weeks of r-hGH DD. Overall, 256 patients received at least 1 dose of study drug and had at least 1 efficacy measurement during the maintenance period (maintenance period modified ITT population). Two hundred nine patients completed the week 36 visit.
Baseline patient characteristics are shown in Table 1. There were no significant differences between treatment groups with respect to demographics; HIV-associated parameters; body composition parameters; or proportions receiving lipid-lowering agents, replacement testosterone, or antiretroviral agents (except nucleoside reverse transcriptase inhibitors [NRTIs], although >93% in both groups were taking NRTIs). A higher proportion of patients in the DD group than in the PL group were receiving antihypertensive agents. There were no significant differences between treatment groups at baseline in efficacy or safety endpoints (Tables 2 and 3).
Efficacy
Changes in Body Composition During Induction Therapy
Absolute changes in body composition parameters in the ITT population from baseline to week 12 are shown in Table 2. The mean change in VAT area was -32.6 cm2 in the DD group and 0.5 cm2 in the PL group (P < 0.001). The mean changes in trunk fat during this period paralleled those in VAT. There were smaller changes in abdominal SAT, limb fat, and total fat, with those in the DD group showing reductions that were larger than those in the PL group (all P < 0.001). In patients who had dorsocervical fat pads (buffalo humps, 74 in the DD group and 23 in the PL group) that could be measured anthropometrically, the DD and PL groups showed reductions in the area of these fat pads from baseline to week 12 (-31.2 cm2 in the DD group vs. -23.7 cm2 in the PL group); the difference between groups did not reach significance (P = 0.091).
In terms of percentage reductions in each of the body composition parameters from baseline to week 12 (Fig. 3), the DD group had a mean 20.3% reduction in VAT area during weeks 1-12, although the PL group showed no decrease (3.6%; P < 0.001). The DD group also had a 20% reduction in trunk fat, 7.1% reduction in abdominal SAT, and 6.0% reduction in limb fat. In contrast, the PL group showed gains in trunk fat, abdominal SAT, and limb fat (1.4%, 1.6%, and 2.8% respectively; P < 0.001 for each between-group difference). At week 12, however, the PL and DD difference in mean percentage reduction in dorsocervical fat area did not reach significance (-27.4% [±34.1%]) in the DD group vs. -13.0% [±39.6%] in the PL group; P = 0.055).
Changes in Body Composition After Induction and Maintenance Therapy
Of those who lost VAT during weeks 1 through 12 and received active maintenance treatment (75 responders in DD-AD-AD group), only 40.3% gained back >50% of the loss, thus meeting the prespecified criterion for maintenance efficacy. At 36 weeks, the DD-AD-AD and DD-PL-PL groups still showed some reduction from baseline in mean VAT (-15.7 cm2 and -7.9 cm2, respectively), but the between-group difference was not statistically significant (see Table 3). In contrast, after 36 weeks, neither group showed a net loss from baseline of abdominal SAT (8.5 cm2 and 5.1 cm2, respectively) or limb fat (0.0 kg and 0.1 kg, respectively).
With respect to mean percentage change from baseline to week 36 in the ITT sample (data not shown), a significant loss of VAT was observed in the DD-AD-AD group (-3.5%; P = 0.018) but not in the DD-PL-PL group (3.1%; P = 0.103). The between-group difference was not statistically significant. Similarly, from baseline to week 36, there was a statistically significant mean percent reduction in trunk fat in the DD-AD-AD group, whereas the DD-PL-PL group showed a small but nonsignificant mean increase in trunk fat.
In the treatment responder subgroup (subjects who lost VAT during the induction phase, 84.8% of the DD group), mean percent changes in VAT from baseline to week 36 within groups also suggested a difference in favor of r-hGH induction-maintenance (-14.7% in the DD-AD-AD group [P < 0.001] vs. 0.8% in the DD-PL-PL group [P = 0.014]), although the difference between the groups was not significant.
As had been seen during induction (baseline to week 12; see Fig. 3), at week 36, r-hGH induction-maintenance therapy (DD-AD-AD group) had proportionately greater effects on VAT and trunk fat than on SAT in the abdomen or limbs; there was no significant reduction in limb fat from baseline to week 36 (see Table 3).
Changes in Lipid Parameters During Induction Therapy
From baseline to week 12, the DD group experienced significant mean absolute (see Table 2) and percent (see Fig. 3) reductions in total cholesterol (-11.4 ± 39.0 mg/dL, -4.1%), non-high-density lipoprotein cholesterol (HDL-C; -13.0 ± 37.1 mg/dL, -6.1%), and low-density lipoprotein cholesterol (LDL-C; -12.5 ± 30.7 mg/dL, -7.1%) and significant mean absolute increases in HDL-C (1.6 ± 7.8 mg/dL, 5.9%) and HDL/LDL ratio (0.7 ± 0.14) (P < 0.001 for each variable listed), whereas subjects in the PL group had only a significant (P < 0.05) mean absolute decrease in LDL-C (-6.4 ± 22.5 mg/dL, -2.9%). Neither group experienced a significant absolute change in serum triglyceride level, but the PL group showed a trend toward an absolute increase (P = 0.074) and had a significant percentage increase (21.1%; P = 0.015). The differences in changes in these variables between groups were significant (P < 0.05) for the absolute increase in HDL-C, decrease in non-HDL-C, and decrease in HDL/LDL ratio as well as for the percent changes in the latter 2 variables (see Fig. 3).
Changes in Lipid Parameters After Induction and Maintenance Therapy
At week 36 (see Table 3), HDL-C remained significantly increased in both groups who had DD induction (4.7 mg/dL or 13.0% in the DD-AD-AD group; 3.8 mg/dL or 12.6% in the DD-PL-PL group; each P < 0.001 relative to baseline levels for absolute and percent changes) and non-HDL-C remained decreased in the DD-AD-AD group (-6.7 mg/dL; P = 0.031 and -2.3%; P = 0.053). In both groups, the HDL/LDL ratio remained increased (0.074 or 23.4% in the DD-AD-AD group; 0.058 or 21.5% in the DD-PL-PL group; all P ≦ 0.001). There were no significant differences between the DD-AD-AD and DD-PL-PL groups in these variables from baseline to week 36. There were no significant changes in LDL-C or triglyceride levels from baseline to week 36 in either group.
Changes in Efficacy Parameters in the Placebo-Placebo-Dosed Daily Group
There were no changes in body composition from baseline to week 12 in the PL-PL-DD group when this group received PL. Data on changes in body composition for the PL-PL-DD group were also available for the period of weeks 12 through 36 (when this group first received PL [weeks 12-24] and then 4 mg/d of r-hGH [weeks 24-36]) but not for the period of weeks 24 through 36, because subjects were not assessed at week 24 by CT or DXA. Statistically significant (P < 0.01) absolute and percent reductions in VAT, trunk fat, SAT, and trunk/limb fat ratio occurred in this group during weeks 12 through 36 (data not shown). The magnitude of the changes from weeks 12 through 36 in the PL-PL-DD group was similar to that seen in the DD group receiving 4 mg/d of r-hGH from baseline to week 12. Absolute and percent changes in lipid variables in this group during active treatment (weeks 24-36) were similar but somewhat smaller than those observed in subjects who started the trial on DD therapy. During weeks 12 through 36, in the PL-PL-DD group, there were also significant (P < 0.02) absolute and percent increases in HDL-C and decreases in non-HDL-C and HDL/LDL ratio (data not shown).
Changes in Concentration of Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-Binding Protein 3 and Other Safety Measurements
From baseline to week 12, there was a significant increase in serum concentration of IGF-I in the DD group (281 ng/mL; P < 0.001), which was greater (P < 0.001) than that in the PL group (23 ng/mL) (see Table 2). There was also a significant increase in mean IGF-BP3 concentration in the DD group (1.2 mg/L; P < 0.001) but no significant increase in the PL group (0.2 mg/L; between-group P < 0.001).
During maintenance (weeks 12-36), IGF-I and IGF-BP3 concentrations declined, but at week 36, they remained significantly elevated relative to baseline levels in the DD-AD-AD group (mean IGF-I = 298 vs. 177 ng/mL, mean IGF-BP3 concentration = 3.0 vs 2.4 mg/L), whereas they returned to baseline in the DD-PL-PL group (Table 3). There were no between-group differences at week 12 or 36 in vital signs, viral load, CD4+ T-cell count, or routine hematologic or clinical chemistry parameters of any clinical significance (see viral load and CD4+ T-cell count in Table 3).
Changes in Safety Variables in the Placebo-Placebo-Dosed Daily Group
The changes in safety variables in the PL-PL-DD group for weeks 24 through 36 were similar in size and pattern to those seen in subjects receiving DD therapy for the period from baseline to week 12, with significant increases in each of the glucose and insulin parameters except HbA1c and significant increases in IGF-I and IGF-BP3 (data not shown).
DISCUSSION
This was the second major trial of r-hGH in HARS that was designed to investigate its ability to reduce visceral fat and associated lipid abnormalities in this disorder. Patients were selected based on the criteria used in the previous trial,5 including anthropometric criteria designating visceral obesity. In both trials, r-hGH at 4 mg/d for 12 weeks reduced VAT (the primary efficacy endpoint) by approximately 20%, with similar reductions in trunk fat.5,12
It is difficult to compare the effects of r-hGH on VAT with those of other agents in HIV-infected patients, because entry criteria vary across trials of different agents. Nevertheless, at 12 weeks in a smaller study of HIV-infected patients with abdominal fat accumulation, a GH-releasing factor, TH9507, given at a dose of 2 mg/d, decreased VAT from baseline by almost as much (-15.7%) as r-hGH did in our clinical trials, but the change for the releasing factor was not significant compared with PL.19
Most studies of thiazolidenedione20-25 and metformin23,26-28 in HIV-associated lipodystrophy found no significant change in VAT. An exception was a small (n = 8) open-label pilot study of rosiglitazone that showed a 21% decrease in 6 to 12 weeks.29 Also, in a metformin study, there was a tendency toward reduction of VAT (P = 0.08 compared with PL) when metformin was administered for 12 weeks, but abdominal SAT decreased in proportion to VAT.26
In the present trial, after 12 weeks of r-hGH, VAT decreased significantly and in a greater proportion than SAT, and trunk fat decreased in a greater proportion than limb fat. These results are more desirable, because patients with HARS may have, or be at risk to develop, subcutaneous lipoatrophy.
The effects of r-hGH at a dosage of 4 mg/d for 12 weeks on trunk fat and VAT in patients with HARS seem to be clinically significant. Because no direct method to assess the clinical significance of VAT loss is available, an indirect method must be used. In weight reduction therapy, treatment is considered clinically significant if it produces a weight loss >5% compared with PL.30 The rationale for this criterion is that even modest weight loss can improve lipid parameters and produce other benefits in people who are obese.31 Therapy with r-hGH does not usually result in weight loss (because r-hGH increases lean tissue as it decreases fat),18 but it reduces fat depots in excess of 5% compared with PL therapy, and it primarily reduces visceral fat, which, in excess, is particularly hazardous to health.32,33
The amount of VAT is a strong correlate of metabolic conditions that substantially increase the risk of CVD among adults in the general population.34,35 These metabolic conditions include elevations in total cholesterol, apolipoprotein B, LDL-C, and triglycerides; decreases in HDL-C; and perturbations of glucose and insulin homeostasis.35 HIV infection is accompanied by significantly increased triglycerides, increased non-HDL-C (rather than increased LDL-C), and decreased HDL-C.36 In HIV-infected patients who are viscerally obese, the effects of visceral obesity on CVD risk may be compounded by the metabolic and other effects of the virus,37 antiretroviral drugs,38 and, if present, lipoatrophy,6,7 thus conferring to this group an especially high risk of developing CVD. VAT accumulation in patients with HIV also has additional adverse effects on health and well-being.8-11
Most patients in both trials of r-hGH in HARS had elevated levels of non-HDL-C, total cholesterol, and/or LDL-C and subnormal concentrations of HDL-C according to accepted guidelines.39 In this trial, baseline non-HDL-C was slightly lower and HDL-C was slightly higher than in the previous trial,5 perhaps because of the more widespread use of atazanavir and tenofovir in this trial (see footnote to Table 1). Atazanavir and tenofovir have fewer adverse effects on the lipid profile than other older antiretrovirals.40-42 Although glucose and insulin abnormalities are also associated with visceral obesity, subjects with abnormal fasting blood glucose and/or 2-hour postload glucose were excluded from the trials.
In this trial, 12 weeks of r-hGH at 4 mg/d significantly decreased non-HDL-C by 6.1% and LDL-C by 7.1%; it increased HDL-C by 5.9%. Corresponding changes in the previous trial were -7.5%, -9.0%, and 11.4%.5 These improvements are consistent with the significant associations reported between VAT and lipoprotein levels in the general population.35 Although the lipid improvements were modest, they are nevertheless important in a population in which dyslipidemia is prevalent5,6 and in which other agents for modifying lipids are less than optimally effective.43,44 In this trial, at baseline, nearly a third of the patients had been receiving lipid-modifying agents (primarily statins and fibrates), yet only 41% were at goal for non-HDL-C at study entry and most (including those receiving such agents) had at least 1 lipid parameter that had an abnormal level at baseline. In prospective studies of patients in the general population who are at cardiovascular risk, every 1% reduction in LDL-C or non-HDL-C is associated with a 1.7% reduction in risk of coronary artery disease45 and every 1-mg/dL increase in HDL-C is associated with a 2% to 3% reduction, independent of the changes in other lipid levels.46
Studies of statin and fibrate therapies in patients with HIV infection have usually shown that they improve non-HDL-C and LDL-C more than r-hGH does, but most did not find a significant increase in HDL-C compared with PL. Moreover, the effects of these drugs on LDL-C and non-HDL-C are generally insufficient to achieve goal levels.43,44 Specifically, in HIV-infected patients, statins have demonstrated mean reductions in non-HDL-C of 9.5% to 18%44,47 and decreases in LDL-C of 14% to 37%;44,48-51 statin-induced increases in HDL-C did not usually reach significance and ranged from 0% to 8%.44,48,51 Fibrates have shown mean reductions in non-HDL-C of 7% to 16.7%,44,52,53 changes in LDL-C of -9.5% to 8% (an increase),27,44 and changes in HDL-C from -1% (a decrease) to 12.6%.27,44,52,53
The lipid-modifying effects of antidiabetic agents and GH-releasing factor in HIV lipodystrophy seem less than those of r-hGH. Rosiglitazone was found to increase total cholesterol in 5 of 6 trials,21,22,25,54,55 LDL-C in 3 trials,54 and triglycerides in 3 trials,21,23,54 although it raised HDL-C in only 1 trial55 and lowered it in another.23 Pioglitazone increased LDL-C and increased HDL-C slightly in 1 trial.24 Metformin did not significantly alter total cholesterol, LDL-C, HDL-C, or non-HDL-C in 4 trials;26-28,55 it increased LDL-C and total cholesterol without increasing HDL-C in another trial25 but decreased triglycerides by 20% to 22% in 2 trials.25,55 In HIV-infected viscerally obese patients, the 2-mg dose of GH-releasing factor TH9507 did not significantly affect LDL-C, HDL-C, or non-HDL-C compared with PL, but it did reduce triglycerides and the total cholesterol/HDL ratio at 12 weeks compared with PL.19
The r-hGH maintenance dose of 2 mg on AD met the predetermined criteria for judging maintenance efficacy (<50% [40.3%] regained >50% of the VAT that they lost), whereas PL maintenance treatment did not. There was some reversal of the improvement in VAT during r-hGH maintenance, however, and even greater reversal on PL maintenance, although at the end of treatment, VAT remained lower than the baseline level after active and PL maintenance. In contrast, the smaller losses of abdominal SAT (-7.1%) and limb fat (-6.0%) seen during induction were completely reversed during maintenance. At the end of the maintenance period, non-HDL-C remained below baseline in the r-hGH maintenance arm but not on PL maintenance. A prolonged effect was seen on HDL-C, which, at week 36, remained increased to greater than baseline on maintenance and PL.
The results of the present trial regarding the safety of r-hGH were similar to those of the previous major trial in HARS5 and indicate an acceptable safety profile. The prevalence of AEs was significantly higher in the r-hGH induction group than on PL in these trials, but approximately 95% of AEs in each trial were rated mild to moderate. There were no differences in the number of SAEs on r-hGH versus PL treatment. Transient arthralgia and swelling sometimes required management by nonsteroid anti-inflammatory drugs (NSAIDS), mild diuretics, or dose reduction. AEs during maintenance were generally less severe and less prevalent than during induction.
The effects of 4 mg/d of r-hGH on glucose and insulin parameters were also similar across the 2 trials, from which glucose-intolerant subjects and diabetics were excluded. In most subjects in these trials, fasting glucose and 120-minute postload glucose levels remained in the normal range throughout the study or returned to normal after mild perturbation during the induction period. HbA1c results were reassuring, because no patient in the study in which this variable was measured (in this trial) showed an increase considered clinically significant. It should be emphasized that this and the previous trial of r-hGH5,12 only included patients with HARS who were normoglycemic at entry, because r-hGH is known to increase serum insulin and glucose concentrations. Other treatment approaches are being explored in diabetic or glucose-intolerant patients in the HARS population, such as GH-releasing factor19 and r-hGH combined with an insulin-sensitizing agent. One pilot study of r-hGH in combination with rosiglitazone in HIV-infected patients reported that the effect of r-hGH on glucose was abrogated by rosiglitazone.56
Another safety issue is that induction therapy with r-hGH significantly increased IGF-I levels in this and the previous trial.5 Some epidemiologists have suggested that in the long term, high levels of IGF-I, alone or in combination with low levels of its binding protein, IGF-BP3, may be associated with an increased risk of malignancy.57-59 Other studies, however, suggest that the increased IGF-BP3 concentrations that accompany increases in IGF-I may reduce cancer risk60 and that high IGF-I may not be a direct marker of the presence of malignancy but might promote the growth of existing cancer cells.61 Patients with cancer, or a history of cancer, were excluded from this trial (except for certain types of skin cancers, such as surgically excised basal cell carcinoma). We are aware of no evidence that rates of cancer increase in HIV-infected patients or others with IGF-I levels similar to those seen in the present or previous trial of r-hGH for HARS, when those levels have been maintained for a short period of time. Supraphysiologic doses of r-hGH that elevate IGF-I levels for short periods have been used to treat HIV-associated wasting18 since 1996. Because HIV-infected patients are at increased risk to develop neoplasms based on currently available data, however, we would not recommend giving GH or GH-like agents to HIV-infected patients with cancer or a history of cancer other than limited cutaneous Kaposi sarcoma or excised basal cell or squamous cell skin cancers.
In addition to the exclusion of patients with current or past cancer and those with diabetes or glucose intolerance, the limitations of this trial included that the maintenance period was only 24 weeks in duration and that only 1 r-hGH maintenance regimen was investigated. Additional research is needed to determine if treatment benefits are retained or diminish during longer maintenance periods and if other maintenance regimens might sustain benefits better than the 2-mg AD dose did in the present study without unacceptable increases in rates of AEs.
In conclusion, our studies found that 4 mg/d of r-hGH administered for 12 weeks as induction therapy for HARS in nondiabetic normoglycemic patients reduced VAT substantially in most of those treated and that most who lost VAT retained a level lower than their baseline level of VAT during 24 weeks of maintenance using 2 mg of r-hGH AD. The VAT reductions were accompanied by beneficial effects on lipids, with few instances of SAEs and generally only transient plasma glucose changes. Thus, r-hGH offers a unique treatment option for patients who have HARS.
|
|
| |
| |
|
|
|