| |
Testing for Chronic Kidney Disease: A Position Statement From the National Kidney Foundation
|
| |
| |
American Jnl of Kidney Diseases Aug 2007
Joseph A. Vassalotti, MD1, Lesley A. Stevens, MD, MS2, Andrew S. Levey, MD2
1 National Kidney Foundation, New York, NY
2 NKF Center for Guideline Development and Implementation, Tufts-New England Medical Center, Boston, MA.
Chronic kidney disease (CKD) is common in the United States. The estimated prevalence of CKD in US adults was 11.7% ± 0.8% in 2000, based on the National Health and Nutrition Examination Survey (NHANES). Global estimates for CKD prevalence are less certain, but recent studies in Europe, Australia, and China suggest a high prevalence. The most common risk factors for CKD include diabetes, hypertension, cardiovascular disease, a family history of CKD, and age greater than 60 years. Major outcomes of CKD include progression to kidney failure, development of complications of impaired kidney function, and increased risk for cardiovascular disease. CKD is usually silent until its late stages, thus many patients with CKD are detected only shortly before the onset of symptomatic kidney failure, when there are few opportunities to prevent adverse outcomes. Earlier detection allows for more time for evaluation and treatment but requires explicit testing strategies for asymptomatic individuals at increased risk. In the majority of patients, CKD can be detected with 2 simple tests: a urine test for the detection of proteinuria and a blood test to estimate the glomerular filtration rate (GFR). These 2 tests facilitate detection of CKD by all physicians by allowing for identification of CKD without first requiring determination of its cause. Understanding the strengths and limitations of CKD testing is critical for appropriate implementation of these recommendations. Application of CKD testing in national and international screening and surveillance programs could improve public health related to CKD. (note from Jules: for HIV-positives baseline testing at patient presentation is I think suggested).
Testing for chronic kidney disease (CKD) facilitates earlier detection, evaluation, and treatment of CKD. Professional and public attention is now focused on CKD as a public health problem. The declaration of World Kidney Day1, 2 and recent conferences by the Kidney Disease Improving Global Outcomes (KDIGO) and Centers for Disease Control (CDC) discuss screening and surveillance programs for CKD.3, 4 Understanding the strengths and limitations of CKD testing is critical for appropriate implementation of these recommendations. The goal of this paper is to discuss CKD testing in clinical practice and its application to public health initiatives, with attention to limitations and appropriate interpretation.5, 6 We emphasize testing both for the presence of kidney damage and for the level of kidney function in adults at increased risk for CKD. CKD testing in children differs in some respects from CKD testing in adults. These differences will be noted but not discussed in detail.
CKD Prevalence, Risk Factors, and Outcomes
CKD is common in the United States. In 2000, approximately 398,000 people (0.2%) were treated by dialysis or transplantation for chronic kidney failure, the end stage of CKD.7 By 2030, this number is expected to rise to more than 2 million people.8 The estimated prevalence of earlier stages of CKD in US adults was 20 million (11.7%) in 2000, based on the National Health and Nutrition Examination Survey (NHANES)9; there are not yet projections for future prevalence of CKD. Worldwide prevalence estimates for kidney failure vary among countries depending upon access to kidney replacement therapy but are growing in most countries.7 Global estimates for earlier stages of CKD are less certain. Some recent studies also suggest a high prevalence of CKD in Europe,10 Australia,11 and China.12
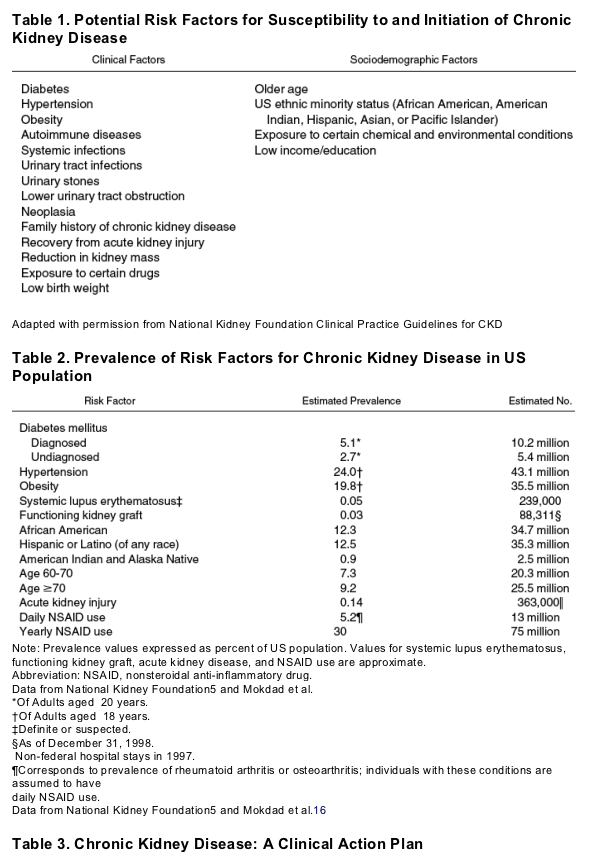
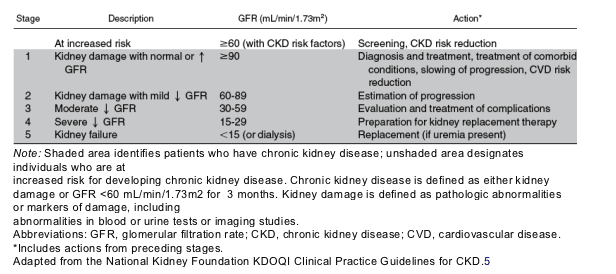
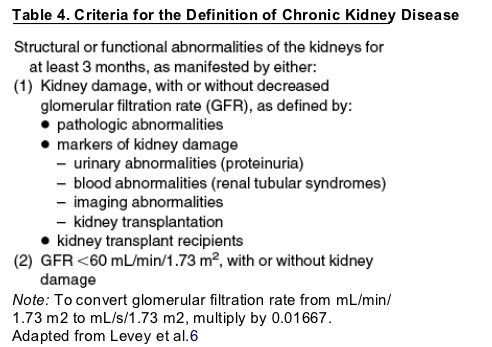
The most common risk factors for CKD include diabetes, hypertension, cardiovascular disease, a family history of CKD, and age greater than 60 years.5, 6, 13, 14, 15 These and other important risk groups are listed in Table 1. The prevalence of each risk factor is estimated in Table 2.5, 16 Major outcomes of CKD include progression to kidney failure, development of complications of impaired kidney function, and increased risk for cardiovascular disease.5, 17, 18, 19 Treatments are now available to reduce the risk of these outcomes, and clinical action plans have now been established for evaluation and treatment of CKD (Table 3).5 CKD is usually silent until its late stages, thus many patients with CKD are detected only shortly before the onset of symptomatic kidney failure when there are few opportunities to prevent adverse outcomes.20 In principle, earlier detection would allow more time for evaluation and treatment but would require explicit testing strategies for asymptomatic individuals at increased risk.
CKD Definition and Testing
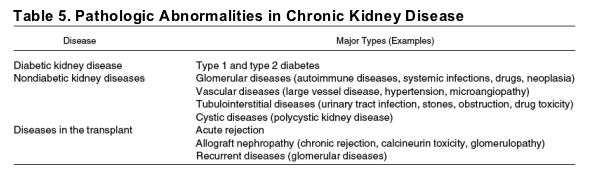
CKD is defined by the presence of kidney damage or glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) for 3 or more months, irrespective of cause (Table 4).5, 6 Markers of kidney damage include abnormalities in the serum or urine, or on imaging studies, and reflect the underlying pathology. Proteinuria is the earliest marker of kidney damage in diabetes, hypertension, and glomerular diseases; thus it is the most common marker of kidney damage in adults.5 GFR is difficult to measure but can be estimated easily from serum creatinine, age, sex, race, or body size.21
Despite this simple definition, CKD is a heterogeneous condition, dependent upon the cause and type (pathology) of kidney disease (Table 5), severity, rate of progression, and comorbid conditions. Nevertheless, in the majority of patients, its presence can be detected with 2 simple tests: a urine test for the detection of proteinuria and a blood test to estimate the GFR. Importantly, these 2 tests facilitate detection of CKD by all physicians, not just nephrologists, by allowing for identification of CKD without first requiring determination of its cause. In this article, we focus on the recommendations for routine testing in the general population at increased risk for CKD. Issues to consider once CKD is detected are mentioned but a complete discussion is beyond the scope of this article. Next, we discuss important issues to consider in ordering and interpreting these tests.
Testing for Kidney Damage
Proteinuria
Proteinuria refers to increased excretion of any urinary protein, including albumin and other serum proteins, and proteins synthesized by the tubule (Tamm-Horsfall protein) or in the lower urinary tract. Albuminuria refers to increased excretion of albumin only. In this article, we refer to testing for proteinuria as tests for detection of proteinuria, including tests for albumin only. We refer to testing for albuminuria as tests for detection of albumin only.
Healthy individuals usually excrete a small amount of protein in the urine. Persistently increased protein excretion is usually a marker of kidney damage. The specific proteins excreted depend upon the type of kidney disease. Increased excretion of albumin is a sensitive and specific marker for CKD due to diabetes, hypertension, and glomerular diseases. Increased excretion of nonalbumin proteins in the absence of albumin occurs in some tubulointerstitial diseases associated with defective reabsorption of filtered, low molecular weight proteins. These diseases are more common in children than adults; therefore, in adults, tests for albumin are preferred because they are more sensitive and specific for the common causes of CKD. In children, tests for total urine protein are preferred, except in children with diabetes, in whom testing for albumin is preferred.
There are many options for measurement of total protein and albumin.5 The most commonly used tests measure total protein (including albumin) or albumin alone. Tests for albuminuria have higher sensitivity and specificity for kidney disease due to diabetes, hypertension, and glomerular diseases than tests for total protein but are more expensive. Assays for total protein and albumin vary across laboratories, which makes it difficult to compare results across laboratories and to determine the normal range. Assays for albumin can be standardized, but there is no "gold standard" for total urine protein, since the composition of urine protein varies among individuals.
In addition, there are multiple methods of urine collection. The 24-hour collection has been regarded as the gold standard but is difficult to implement in routine practice. Measurement of albumin or total protein concentration in a spot sample avoids the need for collection of a timed urine specimen but is affected by the state of hydration. Factoring the concentration of albumin or total protein by urine creatinine concentration (the ratio of albumin-creatinine or total protein-creatinine) eliminates this source of variation but is also affected by urine creatinine excretion rate. Some authors have suggested sex-specific thresholds for the definition of abnormal results to account for differences between men and women in creatinine excretion.9 There are also important differences according to age and race. Table 6 shows the thresholds for the definition of albuminuria and proteinuria according to collection method and sex where appropriate.5 In both adults and children, measurement of the ratio of albumin-creatinine or total protein-creatinine in a spot urine sample is recommended.5
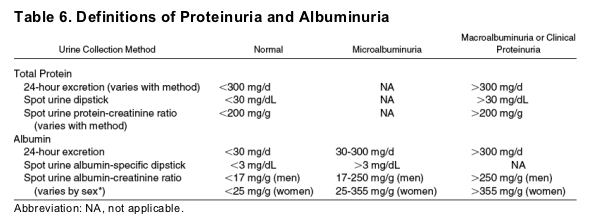
*Sex-specific cut-off values are from a single study. Use of the same cut-off value for men and women leads to higher values of prevalence for women than men. Current recommendations from the American Diabetes Association define cut-off values for spot urine albumin-creatinine ratio for microalbuminuria and albuminuria as 30 mg/g and 300 mg/g, respectively, without regard to sex.
Adapted from National Kidney Foundation KDOQI Clinical Practice Guidelines for CKD
Both total protein and albumin excretion can be increased transiently due to a number of factors, including urinary tract infection, hemodynamic stress (exercise, fever, and congestive heart failure) and transient metabolic perturbations (ketosis and hyperglycemia), which emphasizes the importance of repeated testing to confirm the diagnosis of CKD. Thus, to confirm persistence of proteinuria, 2 of 3 abnormal readings are required over a minimum of 3 months.6Figure 1 shows the algorithm for sequential use of tests in adults and children.22
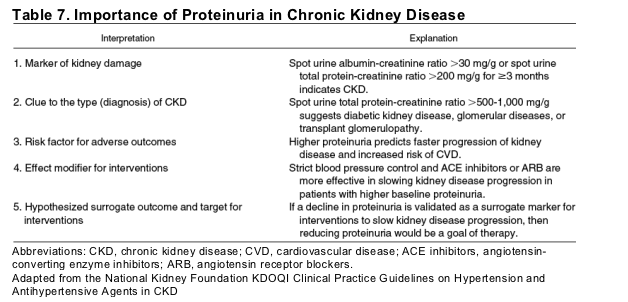
Proteinuria provides important information for evaluation and management of CKD in addition to being a marker for its identification (Table 7).23 Proteinuria is a clue to the cause of CKD (Table 5). Diabetes and other glomerular diseases are associated with higher levels of proteinuria than hypertension and other vascular diseases, tubulointerstitial diseases, or cystic diseases. In addition, regardless of cause of CKD, proteinuria is an important prognostic factor for kidney disease progression and is an indication for use of specific antihypertensive agents (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) to slow progression. Indeed, low levels of albumin (microalbuminuria) are associated with the same relative risk of progression to increased total protein (clinical proteinuria) in patients with and without diabetes.24 Some have hypothesized that proteinuria is a surrogate outcome for kidney disease progression and a target for therapy. Finally, very low levels of albuminuria (below the level defined as microalbuminuria) are associated with increased risk for cardiovascular disease.13
Other Markers of Kidney Damage
Clinical judgment should determine the utility of testing for other markers of kidney damage for detection of CKD, such as imaging studies, other urine or serum markers, or biopsy of the kidney.5 For example, people with family history of polycystic kidney disease should undergo ultrasound examination to detect the presence of cysts, and people with a family history of Alport syndrome should have a urinalysis to detect hematuria. Consultation with a nephrologist is recommended if there are questions about the most appropriate tests for detection of specific diseases other than those mentioned here.
Testing for the Level of Kidney Function: GFR Estimating Equations
GFR is considered the best overall measure of kidney function. Measurement of GFR is cumbersome and expensive, however, and is not usually performed in routine clinical practice; therefore, serum levels of endogenous filtration markers, such as creatinine, have traditionally been used to estimate GFR. Serum creatinine is limited because of variation among laboratories in assays and because its level is also affected by physiologic processes other than GFR, including generation (largely dependent on muscle mass and meat intake), proximal tubular secretion, and extrarenal elimination.15 Estimating equations incorporate creatinine in association with age, sex, race, and body size as surrogates for creatinine generation. One commonly used equation to estimate GFR, the Modification of Diet in Renal Disease (MDRD) Study equation, was developed in patients with CKD and has been re-expressed for use with standardized serum creatinine assay (Table 8).21, 25, 26, 27 The MDRD Study equation has now been evaluated in numerous populations, including African Americans, Europeans, and Asians, diabetic and nondiabetic patients with and without kidney disease, kidney transplant recipients, and potential kidney donors. These studies have shown that in American or European populations, the MDRD Study equation has reasonable accuracy in nonhospitalized patients known to have CKD, regardless of diagnosis.28 It is likely that the MDRD Study equation will not perform as well in other populations due to differences in diet or muscle mass. Two recent evaluations of the MDRD Study equation in China and Japan showed conflicting results, with the first study showing an underestimation of the measured GFR, and the second showing an overestimation.29, 30 It is not known whether these conflicting results are due to differences in creatinine assay, differences in GFR measurement procedures, or differences among populations in creatinine generation.

Note: Age is expressed in years; weight is expressed in kilograms; body surface area is calculated as W 0.425 x H 0.725 x 0.007184 /1.73 m2.
To convert serum creatinine in mg/dL to mol/L, multiply by 88.4.
Abbreviations: MDRD, Modification of Diet in Renal Disease; Scr, serum creatinine; Ccr, creatinine clearance; BSA, body surface area.
In all populations studied so far, the MDRD Study equation has been reported to have lower accuracy in populations without kidney disease than in patients with CKD, such as young patients with type 1 diabetes without microalbuminuria or people undergoing evaluation for kidney donation.31, 32, 33 There are several possible explanations for decreased accuracy of higher GFR estimates, including (1) interlaboratory variation in the calibration of serum creatinine assay, which has a larger effect at higher GFR levels34; (2) greater biologic variation and errors in measurement of GFR at higher range of GFR; and (3) limitations of applying an equation developed in people with CKD to a population predominantly without CKD.15 The limitations discussed above are relevant for all GFR estimating equations.
The MDRD Study equation has not yet been validated in children (age less than 18 years), pregnant women, adults older than 85 years, or in some racial or ethnic subgroups in the United States, such as Asians, Native Americans, and Hispanics. Furthermore, effects of diet and medications on serum creatinine are not accounted for in the MDRD Study equation.35
Many studies have compared the performance of the MDRD Study and the Cockcroft-Gault equations (Table 8). In some of these studies, the MDRD Study equation was more accurate than the Cockcroft-Gault equation. Other studies demonstrated similar performance. The Cockcroft-Gault equation appears to be less accurate than the MDRD Study equation specifically in older and obese people.15 The Cockcroft-Gault equation has not been re-expressed for use with standardized serum creatinine assays. With planned standardization of the serum creatinine assay, GFR estimates based on the Cockcroft-Gault equation will likely routinely overestimate measured GFR and become less useful.36
Interpretation of CKD Tests and Integration into the CKD Clinical Action Plan
Interpretation of CKD Tests
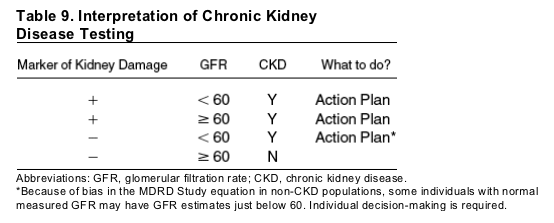
Persistent proteinuria or reduction in GFR to below 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) is defined as CKD. The lesser accuracy of current estimating equations at higher levels of GFR may make it difficult to interpret GFR estimates near 60 mL/min/1.73m2 (1.0 mL/s/1.73 m2) in some people. In this range, interpretation of GFR estimates depends on the results of tests for markers of kidney damage and the clinical context (Table 9). Patients with proteinuria have CKD even if GFR estimates are 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) or greater. Patients without markers of kidney damage and with GFR estimates of 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) or greater are unlikely to have CKD. There will be some uncertainty for patients without markers of kidney damage and with GFR estimates just below 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2). Some patients will have measured GFR less than 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) and would therefore have CKD (a "true-positive" test result). Others may have a measured GFR above 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) and therefore would not have CKD (a false-positive test result). Clinical interpretation and decision-making will depend on the patient's risk for CKD. An estimated GFR of less than 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) is more likely to be a true-positive result in a patient at increased risk for CKD (ie, a patient with hypertension, diabetes, cardiovascular disease, or a family history of CKD) than in a patient not at increased risk. Current guidelines recommend routine CKD testing in patients at increased risk for CKD. Restricting routine testing to patients at increased risk for CKD is likely to reduce false-positive results and enable more efficient use of resources.5, 6, 15, 37 In patients at increased risk for CKD and with an estimated GFR less than 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2), it is appropriate for clinicians to proceed with the CKD clinical action plan (Table 3). In patients not at increased risk for CKD and with an estimated GFR less than 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2), clinicians may decide to defer further evaluation for CKD. Nonetheless, it may be prudent to monitor estimated GFR more frequently, adjust the dosage of medications that are excreted by the kidney, and avoid medications toxic to the kidney.
Clinical Action Plan for CKD
CKD can be identified in most people using the 2 simple tests described above; however, the evaluation and management should be individualized according to the cause and type (pathology) of kidney disease, severity, rate of progression, and comorbid conditions. Individualized care should begin with a clinical action plan tailored to the stage of disease (Table 3). Thorough evaluation of CKD includes assessment of its severity, markers of kidney damage, cause, and complications.
Referral to Nephrologists
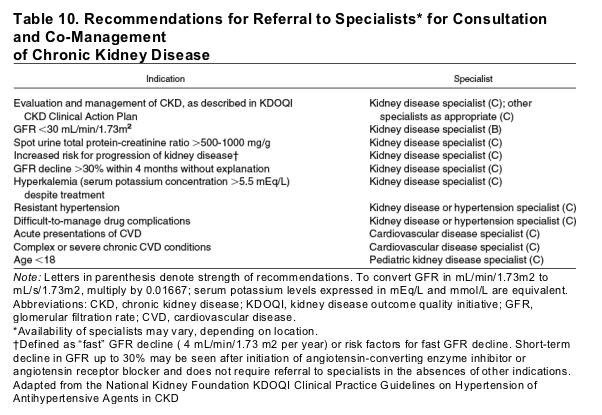
Patients with CKD stage 4 should be referred to a nephrologist for co-management with their primary care physician or other specialists.5 Late nephrology referral before dialysis initiation is associated with increased morbidity and mortality.20, 38 It is thought that early planning for kidney replacement therapy and increased attention to CKD complications and cardiovascular disease risk factors may improve overall health care status; however, there are many other reasons for consultation with a nephrologist earlier in the course of disease (Table 10), such as uncertainty in diagnosis or treatment, total urine protein greater than 1 g/d, rapid GFR decline (>30% within 4 months without explanation), and difficult to control hypertension or hyperkalemia.23 In general, consultation should be sought if the clinical action cannot be prepared, or if the prescribed evaluation or the recommended treatment cannot be carried out.5 Nephrology referral may not always be appropriate for patients with terminal illnesses such as severe heart failure, liver failure, or metastatic cancer in whom care is focused on palliation with no plans for kidney replacement therapy.
Limitations
There are 3 main limitations to the current testing recommendations. First, there is a variable rate of false-positive tests for both urine protein and estimated GFR, depending upon the population screened. Repeated measurements, with confirmation of persistence of abnormal results over 3 months, will diminish the false-positive rate of urine protein testing. Attention to the clinical setting will facilitate proper interpretation of both urine protein and estimated GFR. Second, there is uncertainty about the implications for patient outcomes associated with CKD, depending on the clinical setting. Although studies have documented increased risk for all-cause mortality, clinical cardiovascular disease events, complications of low GFR, and progression to kidney failure with reduced GFR and with proteinuria, a prediction for an individual person is difficult to quantify. At present, there is no equivalent to a Framingham risk equation for clinical cardiovascular disease events to relate the risk for any or all CKD outcomes to the combination of level of GFR and proteinuria. Finally, the thresholds for abnormal and normal levels of GFR and urine protein were derived primarily from the studies of adults in the United States and Europe and may not be applicable to all ages or different geographic, racial, or ethnic groups.
The limitations related to the measurement and interpretation of CKD tests are actively being addressed. The laboratory workgroup of the National Kidney Disease Education Program (NKDEP) of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) has developed a process to standardize the serum creatinine assay to a high-quality reference standard of the National Institute of Standards and Technology (NIST) with the goal of implementing assay standardization in all clinical laboratories.39 This work is critical to optimizing accuracy of GFR estimating equations and is expected to be completed by 2008. The workgroup is now addressing the challenges of standardization of albumin, protein, and creatinine measurements in urine. This will be a major step forward in interpretation of urine protein tests and will facilitate development of uniform reporting formats and normal values for age, sex, and race.40
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), a research team supported by the NIDDK, is developing improved GFR estimating equations from creatinine, cystatin (an alternative endogenous filtration marker not related to muscle mass), or both. One of the key goals is to improve the accuracy of GFR estimates near 60 mL/min/1.73 m2 (1.0 mL/s/1.73 m2) in order to improve identification of CKD.
Application to Public Health Programs
The strategy of screening for CKD by testing both for markers of kidney damage and for the level of kidney function has now been applied in NHANES and in the National Kidney Foundation's Kidney Early Evaluation Program (KEEP). NHANES is an ongoing survey of a stratified random sample of the noninstitutionalized US population. Data from more than 25,000 people since 1988 has been critical to demonstrate prevalence of CKD, associations with comorbid conditions, and trends over time.9, 41 In the population with CKD, only 2.9% of women and 17.9% of men reported being aware of the diagnosis.9 KEEP is a voluntary, community-based screening program, which has been offered free of charge to people at increased risk for CKD since 1997. More than 90,000 people have been screened for CKD as of March 1, 2007. In this high-risk population, 29% were observed to have CKD. Of the people identified to have CKD, only 6.7% of patients were aware of having CKD, which is consistent with data from NHANES.42 Both activities highlight the burden of CKD in a population largely unaware of its presence, emphasizing the need for widespread public health activities. The CDC is currently working with the National Kidney Foundation to implement a KEEP-like model in a state-level demonstration project for screening individuals at risk for CKD. Application of CKD testing in national and international screening and surveillance programs could improve public health related to CKD.3, 4
Acknowledgements
This paper has been approved as the official position of the National Kidney Foundation by the Scientific Advisory Board and Board of Directors.
Support: Dr Vassalotti is the Chief Medical Officer of the National Kidney Foundation. Dr Stevens is Program Director, Implementation, at the National Kidney Foundation Center for Guideline Development and Implementation at Tufts-New England Medical Center; Dr Levey is Director of that center and Editor-in-Chief of AJKD.
Financial Disclosure: Dr Levey acknowledges receipt of grants and contracts from the National Institute of Diabetes, Digestive and Kidney Disease and Amgen, but there are no conflicts of interest with the subject of this article. Dr Stevens acknowledges receipt of grants and contracts from the National Institute of Diabetes, Digestive and Kidney Disease and the American Society for Nephrology, as well as lecture fees from Quest Diagnostics, but there are no conflicts of interest with the subject of this article. Dr Vassalotti reports having received grant support from the Centers for Disease Control and Prevention, but there are no conflicts of interest with the subject of this article.
|
|
| |
| |
|
|
|