| |
Raltegravir FDA Clinical Development Summary
|
| |
| |
This NDA contains clinical data collected primarily from four clinical studies, including the two pivotal studies Protocol 018 and Protocol 019. Protocols 018 and 019 are international, multi-center, double-blind, randomized, placebo-controlled trials comparing raltegravir in combination with optimized background therapy (OBT) to OBT alone in highly treatment-experienced HIV-infected subjects. The studies were identical except for the location of the study sites. Protocol 018 was conducted in Europe, Asia/Pacific, and South America, while Protocol 019 was conducted in North and South America. Eligible subjects were HIV-1 infected patients who had failed therapy as documented by HIV RNA >1,000 copies/mL while on stable therapy and documented resistance to at least 1 drug in each of 3 classes of licensed oral ARVs (NNRTI, NRTI, and PI).
Several supportive studies were also submitted, including Protocol 004, a dose-finding study in treatment-naive patients and Protocol 005, a dose-finding study in treatment-experienced patients that evaluated doses of 200 mg, 400 mg, and 600 mg of raltegravir versus OBT for 48 weeks. Dose selection for Phase 3 was based on Week 24 study data.
A pediatric study is currently underway, but no pediatric data were submitted in support of this NDA. Other ongoing studies include expanded access Protocol 023 and a large Phase 3 trial in treatment-naive patients, Protocol 021.
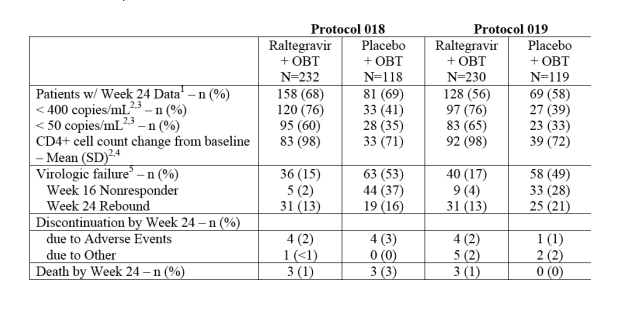
TABLE 3 - Week 24 Outcomes
At week 24 (protocol 18), 76% <400 in Raltegravir+OBT arm, 41% in placebo+OBT; 60% <50 in RALT+OBT+OBT. Week 16 nonresponder: 2% RALT, 37% placebo; week 24 rebound: 13% RALT, 16% placebo (protocol 19 had similar results).
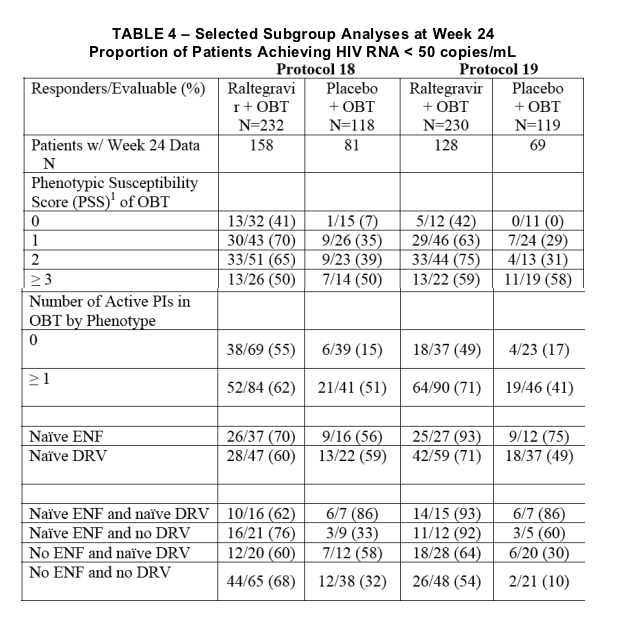
1PSS score was defined as the total oral ARVs in OBT to which a patient's viral isolate showed phenotypic sensitivity based on phenotypic resistance test. Enfuvirtide use in OBT in enfuvirtide-naive patients was counted as one active drug in OBT and added to the PSS. Darunavir use in OBT in darunavir-naive patients was counted as one active drug in OBT and added to the PSS.
|
|
| |
| |
|
|
|