| |
Electrocardiographic changes in HIV-infected, drug-experienced patients being treated with atazanavir
[Research Letters]
|
| |
| |
AIDS:Volume 21(12)31 July 2007p 1648-1651
Gianotti, Nicolaa; Guffanti, Monicaa; Galli, Lauraa; Margonato, Albertob; Chiaravalli, Giuliaa; Bigoloni, Albaa; Lazzarin, Adrianoa,c; Castagna, Antonellaa
aDivisione di Malattie Infettive, Istituto Scientifico San Raffaele, Milan, Italy
bDipartimento di Cardiologia, Istituto Scientifico San Raffaele, Milan, Italy
cUniversita Vita-Salute San Raffaele, Milan, Italy.
Abstract
The QRS interval of 56 out of 75 (74.7%) HIV-infected, drug-experienced patients (66.7% men) increased during treatment with boosted or unboosted atazanavir by a median 5 ms (interquartile range 0-9; P < 0.0001); the PR and the QTc intervals did not change significantly. New asymptomatic bundle branch blocks were observed in four patients; one subject with a baseline first-degree atrioventricular block developed symptomatic bradyarrhythmia while receiving atenolol. The electrocardiographic monitoring of patients treated with atazanavir seems advisable.
From Reyataz Label:
Effects on Electrocardiogram
Concentration- and dose-dependent prolongation of the PR interval in the electrocardiogram has been observed in healthy volunteers receiving atazanavir. In a placebo-controlled study (AI424-076), the mean (±SD) maximum change in PR interval from the predose value was 24 (±15) msec following oral dosing with 400 mg of atazanavir (n=65) compared to 13 (±11) msec following dosing with placebo (n=67). The PR interval prolongations in this study were asymptomatic. There is limited information on the potential for a pharmacodynamic interaction in humans between atazanavir and other drugs that prolong the PR interval of the electrocardiogram. (See WARNINGS.)
--Electrocardiographic effects of atazanavir were determined in a clinical pharmacology study of 72 healthy subjects. Oral doses of 400 mg and 800 mg were compared with placebo; there was no concentration-dependent effect of atazanavir on the QTc interval (using Fridericia's correction). In 1793 HIV-infected patients receiving antiretroviral regimens, QTc prolongation was comparable in the atazanavir and comparator regimens. No atazanavir-treated healthy subject or HIV-infected patient had a QTc interval >500 msec.
PR Interval Prolongation
Atazanavir has been shown to prolong the PR interval of the electrocardiogram in some patients. In healthy volunteers and in patients, abnormalities in atrioventricular (AV) conduction were asymptomatic and generally limited to first-degree AV block. There have been rare reports of second-degree AV block and other conduction abnormalities and no reports of third-degree AV block (see OVERDOSAGE).
--In clinical trials, asymptomatic first-degree AV block was observed in 5.9% of atazanavir-treated patients (n=920), 5.2% of lopinavir/ritonavir-treated patients (n=252), 10.4% of nelfinavir-treated patients (n=48), and 3.0% of efavirenz-treated patients (n=329). In Study AI424-045, asymptomatic first-degree AV block was observed in 5% (6/118) of atazanavir/ritonavir-treated patients and 5% (6/116) of lopinavir/ritonavir-treated patients who had on-study electrocardiogram measurements. Because of limited clinical experience, atazanavir should be used with caution in patients with preexisting conduction system disease (eg, marked first-degree AV block or second- or third-degree AV block). (See CLINICAL PHARMACOLOGY: Effects on Electrocardiogram.)
--In a pharmacokinetic study between atazanavir 400 mg once daily and diltiazem 180 mg once daily, a CYP3A substrate, there was a 2-fold increase in the diltiazem plasma concentration and an additive effect on the PR interval. When used in combination with atazanavir, a dose reduction of diltiazem by one half should be considered and ECG monitoring is recommended. In a pharmacokinetic study between atazanavir 400 mg once daily and atenolol 50 mg once daily, there was no substantial additive effect of atazanavir and atenolol on the PR interval. When used in combination with atazanavir, there
is no need to adjust the dose of atenolol. (See PRECAUTIONS: Drug Interactions.)
--Pharmacokinetic studies between atazanavir and other drugs that prolong the PR interval including beta blockers (other than atenolol), verapamil, and digoxin have not been performed. An additive effect of atazanavir and these drugs cannot be excluded; therefore, caution should be exercised when atazanavir is given concurrently with these drugs, especially those that are metabolized by CYP3A (eg, verapamil). (See PRECAUTIONS: Drug Interactions.)
Cardiomyopathies [1-5] and QTc prolongation [6-9] have been associated with HIV infection and treatment with antiretroviral agents. Asymptomatic prolongations of the PR and QTc intervals have been observed in healthy volunteers and HIV-infected patients receiving atazanavir [10,11], but the effect of atazanavir on the QRS interval has not been specifically investigated in these studies. The aim of the present study was to investigate heart conduction disturbances in HIV-infected, drug-experienced patients treated with boosted or unboosted atazanavir in combination with other antiretroviral agents.
The analysis included patients enrolled in the atazanavir expanded access programme AI424-900 for whom 12-lead electrocardiograms (ECG) were available at baseline and study end. The expanded access programme exclusion criteria were a heart rate of less than 40 bpm, pause length of more than 3 s, second or third-degree atrioventricular block, and QTc interval of more than 450 ms (men) or more than 470 ms (women). The local Ethics Committee approved the study, and all of the patients gave their informed consent.
The ECG were recorded using the same HP-PageWriter 200 M1771A (1996; Hewlett Packard, Andover, Massachusetts, USA); the intervals were automatically calculated, and all of the ECG were reviewed by a single blinded cardiologist.
The results are expressed as median values (interquartile range) or frequencies (%) as appropriate. Continuous variables were analysed using the non-parametric Mann-Whitney rank sum test (to compare the values of independent distributions), Wilcoxon's signed rank test (to assess significant variations from baseline), and Spearman's rank correlation coefficient (to assess linear correlations). The chi-square or Fisher's exact test were used to detect significant relationships between discrete variables. In the multivariable analysis, the general linear model was applied to estimate the independent contribution of some variables (exposure to boosted atazanavir compared with exposure only to unboosted atazanavir, the baseline QRS interval, the baseline CD4 T-lymphocyte count and log10 HIV RNA) to predict a change in the QRS interval. All of the statistical tests were two-sided at the 5% level, and performed using SAS software, v.8.2 (SAS Institute Inc., Cary, North Carolina, USA).
Seventy-five of the 128 HIV-infected, drug-experienced patients enrolled in the expanded access programme at our centre between November 2002 and December 2004 (66.7% men) satisfied the inclusion criteria for the analysis.
At baseline, they were aged 44 years (39-53), weighed 67 kg (55-76), and had CD4 cell counts of 326 cells/ƒÊl (242-518) and 2.57 log10 copies of HIV-RNA/ml (1.69-4.46). Their serum glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, and kalium levels were, respectively, 89 mg/dl (83-99), 191 mg/dl (157-228), 40 mg/dl (34-49), 120 mg/dl (87-149), 185 mg/dl (115-259), and 4.1 mmol/l (3.96-4.3).
The baseline heart rate was 71 bpm (63-77), and the PR, QRS and QTc intervals were, respectively, 161 (149-173), 87 (82-94) and 402 (387-414) ms. Six patients (8%) had a QRS interval of more than 100 ms.
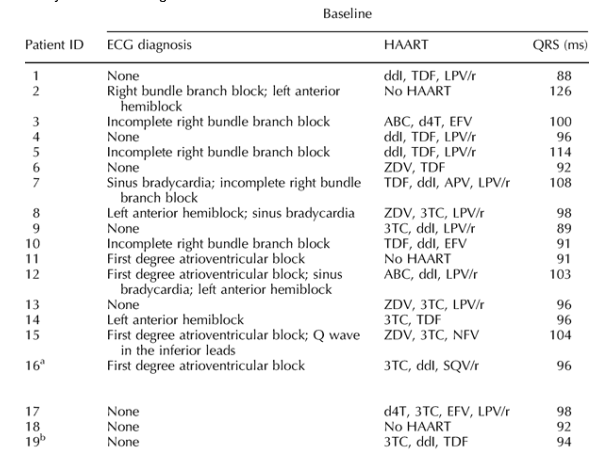
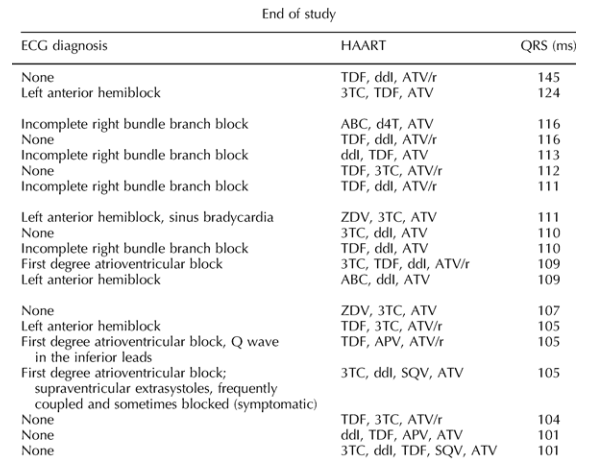
After 51 weeks of follow-up (36-89.9), the median QRS interval was significantly longer [91; 85-101; variation 5 ms (0-9); P < 0.0001] than at baseline, having increased in 56 patients (74.7%). Five of the six patients with a baseline QRS intervals greater than 100 had an interval of more than 100 at the end of the study, as did 14 new subjects (18.7%; difference from baseline 17.3%; 95% confidence interval 7.2-19.9%; P = 0.0008; Table 1).
The PR interval increased to 161 ms (149-174), with a variation of 4 ms (from -5 to 10; P = 0.07); the QTc interval and heart rate were not statistically different from baseline. Serum kalium levels increased significantly to 4.25 mmol/l (4.04-4.49; variation 0.06; from -0.14 to 0.38; P = 0.014) but at the end of the study, only two patients had greater than 5 mmol/l; there was no correlation between baseline kalium levels, kalium changes or the highest recorded kalium value and the changes in PR, QRS or QTc.
Twenty-one subjects (28%) were exposed to boosted atazanavir for 46.9 weeks (26.9-51.3). The QRS interval increased by 6 ms (3-9; P < 0.0001) in the patients exposed to boosted atazanavir and 4 ms (from -1 to 8; P < 0.0001) in those exposed only to unboosted atazanavir. The QRS interval increased in 20 out of 21 subjects (95.2%) exposed to boosted atazanavir compared with 36 out of 54 (66.7%) of those exposed only to unboosted atazanavir (P = 0.016), but the changes correlated only with the end-of-study interval (r = 0.45; P < 0.0001). There was no correlation between the QRS changes and heart rate, PR, QTc, RR changes or the duration of exposure to boosted or unboosted atazanavir. At multivariable analysis, the only independent predictor of the QRS interval change was the exposure to boosted atazanavir (ƒÀ = 5.79, P = 0.013).
New bundle branch blocks were observed in four patients (two with left anterior hemiblock and two an incomplete right bundle branch block); the right bundle branch block observed at baseline in one patient was not detected at the study end. The QRS prolongations were always asymptomatic, and there were no clinical events related to delayed ventricular conduction during treatment. One subject developed an asymptomatic first-degree atrioventricular block. One with a baseline first-degree atrioventricular block developed symptomatic bradyarrhythmia while receiving atenolol; the symptoms recovered after the atenolol dose was reduced and atazanavir discontinued.
After a one-year follow-up, there were no significant changes in PR, QTc or RR intervals in our case series. Patients with a prolonged QTc interval at baseline were, however, excluded from the study and there was a clear trend towards PR prolongation, which suggests that the results may have been different in a larger or unselected patient sample.
On the contrary, there was a significant increase in QRS intervals, consistent with the findings of Busti et al. [12] in 21 HIV-infected men. The increase was larger and more frequent among the patients receiving boosted atazanavir, and was not related to hyperkalemia or the use of cardiological drugs delaying intraventricular conduction. No correlation was found between QRS interval changes and the duration of boosted atazanavir exposure, however, and the increase was significant in the patients receiving boosted or unboosted atazanavir; the present study does not therefore demonstrate a dose-dependent effect. Furthermore, it does not rule out the possible role of antiretroviral drugs other than atazanavir in increasing the QRS interval. In some patients (e.g. numbers 1, 3, 4, 8, 9, 10, 13 and 14) the backbone was unchanged and only atazanavir was introduced in the new regimen, but large increases in the QRS interval still occurred; these individual cases seem very suggestive of a possible role of atazanavir as leading cause of the increase in the QRS interval.
Symptomatic bradyarrhythmia associated with a worsening first-degree atrioventricular block occurred in one patient treated with beta-blockers, and so it is not known whether atazanavir was the main cause of these alterations.
The clinical implications of our findings are unclear, but it seems wise to suggest that all patients receiving atazanavir should undergo regular ECG monitoring, particularly when atazanavir is boosted with ritonavir or combined with drugs delaying heart conduction.
Table 1. Electrocardiographic abnormalities, cardiological and antiretroviral therapies in HIV-infected patients treated with atazanavir, and with an end-of-study QRS interval greater than 100 ms.
Acknowledgements
The authors wish to thank Bristol-Myers Squibb Italia for providing Reyataz (Expanded Access Program AI424-900).
Sponsorship: N.G., A.L. and A.C. are recipients of financial support for research and educational programmes from Glaxo-Smith Kline, Bristol-Myers Squibb, Abbott, Gilead Sciences, Roche, Boehringer-Ingelheim, Pfizer, and Virco (Johnson & Johnson). M.G. is a recipient of financial support for research and educational programmes from Glaxo-Smith Kline, Bristol-Myers Squibb, Abbott, Gilead Sciences, and Roche. L.G., A.M., G.C. and A.B. have no potential conflict of interest to declare.
References
1. Grody WW, Cheng L, Lewis W. Infection of the heart by the human immunodeficiency virus. Am J Cardiol 1990; 66:203-206.
[Medline Link] [CrossRef] [Context Link]
2. Herskowitz A, Willoughby S, Wu TC, Beschorner WE, Neumann DA, Rose NR, et al. Immunopathogenesis of HIV-1-associated cardiomyopathy. Clin Immunol Immunopathol 1993; 68:234-241.
[Medline Link] [CrossRef] [Context Link]
3. Kaul S, Fishbein MC, Siegel RJ. Cardiac manifestations of acquired immune deficiency syndrome: a 1991 update. Am Heart J 1991; 122:535-544.
[Medline Link] [CrossRef] [Context Link]
4. Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al, Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV multicenter study. Pediatric pulmonary and cardiac complications of vertically transmitted HIV infection (P2C2 HIV) study group. Circulation 1998; 97:1246-1256.
[Context Link]
5. Stewart JM, Kaul A, Gromisch DS, Reyes E, Woolf PK, Gowitz MH. Symptomatic cardiac dysfunction in children with human immunodeficiency virus infection. Am Heart J 1989; 117:140-144.
[Medline Link] [CrossRef] [Context Link]
6. Castillo R, Pedalino RP, El-Sherif N, Turitto G. Efavirenz-associated QT prolongation and torsade de pointes arrhythmia. Ann Pharmacother 2002; 36:1006-1008.
[Medline Link] [CrossRef] [Context Link]
7. Sani MU, Okeahialam BN. QTc interval prolongation in patients with HIV and AIDS. J Natl Med Assoc 2005; 97:1657-1661.
[Context Link]
8. Anson BD, Weaver JG, Ackerman MJ, Akinsete O, Henry K, January CT, et al. Blockade of HERG channels by HIV protease inhibitors. Lancet 2005; 365:682-686.
[Medline Link] [CrossRef] [Context Link]
9. Nordin C, Kohli A, Beca S, Zaharia V, Grant T, Leider J, et al. Importance of hepatitis C coinfection in the development of QT prolongation in HIV-infected patients. J Electrocardiol 2006; 39:199-205.
[Medline Link] [CrossRef] [Context Link]
10. Bristol-Myers Squibb Company. Reyataz (atazanavir sulfate) package insert, revised February 2006. Princeton, NJ, USA. Available at: http://www.bms.com/cgi-bin/anybin.pl?sql=select+PPI+from+TB_PRODUCT_PPI+where+PPI_SEQ=103 . Accessed: 13 June 2006.
[Context Link]
11. Bristol-Myers Squibb Company. BMS-232632: Atazanavir briefing document May 2003. Available at: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3950B1_01_BristolMyersSquibb-atazanavir.pdf . Accessed: 13 June 2006.
[Context Link]
12. Busti AJ, Tsikouris JP, Peeters MJ, Das SR, Canham RM, Abdullah SM, et al. A prospective evaluation of the effect of atazanavir on the QTc interval and QTc dispersion in HIV-positive patients. HIV Med 2006; 7:317-322.
[Context Link]
|
|
| |
| |
|
|
|