| |
New Study: HIV+ Blacks Have High Rates of End-Stage Renal Disease in USA VA
|
| |
| |
Racial Differences in End-Stage Renal Disease Rates in
HIV Infection versus Diabetes
Journal of the American Society of Nephrology
JASN Express. Published on October 17, 2007 as doi: 10.1681/ASN.2007040402
Andy I. Choi,* Rudolph A. Rodriguez, Peter Bacchetti, Daniel Bertenthal,
Paul A. Volberding, and Ann M. O'Hare
*Department of Medicine, San Francisco General Hospital, Departments of Medicine and Epidemiology and Biostatistics, University of California, San Francisco, and Department of Medicine and VA Research Enhancement
Award Program, San Francisco VA Medical Center, San Francisco, California; and Department of Medicine, VA Puget Sound Healthcare System and University of Washington, Seattle, Washington
"In this national cohort of veterans, the incidence of ESRD among black patients with HIV was approximately 10 times that among white patients with HIV, almost twice that among white patients with diabetes, and similar to that among black patients with diabetes. These findings demonstrate that kidney disease in the setting of HIV infection disproportionately affects black individuals and emphasize the importance of defining strategies to understand, prevent, and treat CKD among black individuals who are infected with HIV"
ABSTRACT
Few studies have compared the incidence of end-stage renal disease (ESRD) among individuals with the human immunodeficiency virus (HIV) and diabetes. We followed a national sample of 2,015,891 US veterans over a median peroid of 3.7 years for progression to ESRD. The age- and sex-adjusted incidence
of ESRD (per 1000 person-years) among HIV-infected black patients was nearly an order of magnitude higher than among HIV-positive white patients, almost twice that of diabetic whites, and similar to that among diabetic blacks.
In multivariate Cox proportional hazards analysis, diabetes was associated with
an increased risk of ESRD among white patients, but HIV was not. Among black individuals, however, both HIV and diabetes conferred a similar increase in the risk of ESRD (4- to 5-fold increase compared to white individuals without HIV or diabetes). HIV and diabetes carry a similar risk of ESRD among black patients, highlighting the importance of developing strategies to prevent and treat renal disease among HIV-infected black individuals.
These findings demonstrate that race is a critical determinant of ESRD risk among HIV-infected patients and underscore the importance of HIV as a risk factor for ESRD among black individuals.
Chronic kidney disease (CKD) is a growing problem among HIV-infected individuals.1 The number of HIV-infected patients with ESRD doubled between
1995 and 2000, and HIV-associated nephropathy is the third leading cause of ESRD among black individuals in the United States who are aged 20 to 64 yr.2 Although the prevalence of ESRD in the HIV-infected population is projected to increase,3 very few studies have attempted to define the importance of HIV as a risk factor for progression of renal disease. Because of the rarity of ESRD as a clinical outcome, previous studies relied on surrogate measures for renal disease progression, such as increases in creatinine or the development of proteinuria.4,5 Furthermore, few studies have provided contemporary estimates of the risk for ESRD among HIV-infected patients since the advent of antiretroviral therapy.3,6 To better understand the effect of HIV on progression of renal disease, we examined the incidence of ESRD in a national cohort of US veterans. To place our results in context, we compared the risk for ESRD associated with HIV with that of diabetes, a widely recognized risk factor for ESRD.
RESULTS
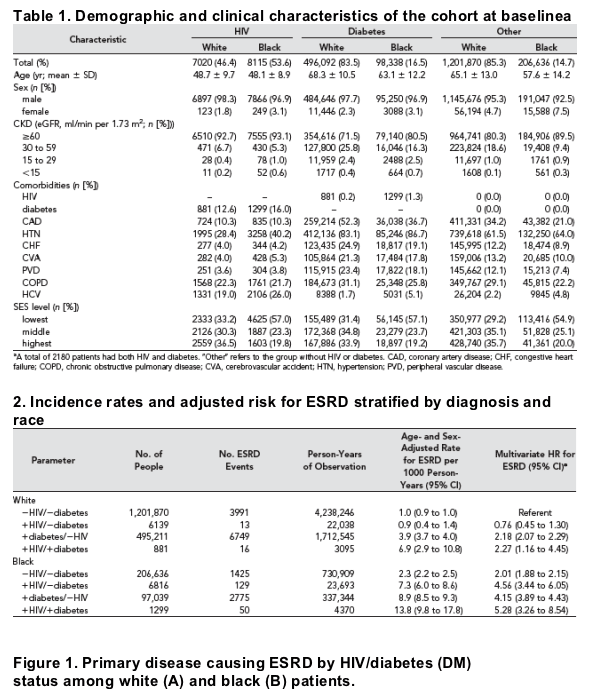
A total of 15,135 (0.8%) patients in the cohort had HIV, 594,430 (29.5%) carried a diagnosis of diabetes, and 1,408,506 (69.9%) did not have either condition
(Table 1). Among patients with HIV, 53.6% (n = 8115) were black. In contrast, black patients composed only 16.5% (n =98,338) of those with diabetes and 14.7% (n =206,636) of those with neither condition. Compared with the referent group without HIV or diabetes (mean age 64.0 yr; SD +/-13.4), patients with HIV were younger (mean age 48.4 yr; SD+/-9.3), and those with diabetes
were older (mean age 67.4 yr; SD+/-11.0).
At baseline, 7.1% (n =1070) of HIV-infected patients, 27.0% (n =160,674) of those with diabetes, and 18.4% (n =258,859) of those with neither condition had CKD defined as an estimated GFR (eGFR) <60 ml/min per 1.73m2. The prevalence of CKD was approximately equal between white (7.3%) and black (6.9%) patients in the HIV-infected group; however, CKD was much more common in white versus black individuals in the groups with diabetes (28.5 versus 19.5%) and without HIV or diabetes (19.7 versus 10.5%). Whereas most comorbid conditions were far less prevalent among patients with HIV than among those with diabetes, hepatitis C virus (HCV) was found to be more common among patients with HIV. Compared with the other groups, a larger percentage (46.0%) of HIV-infected patients were in the lowest tertile of socioeconomic
status (SES).
The median observation time was 3.7 yr (interquartile range 3.4 to 4.0 yr). A total of 15,586 cases of ESRD occurred during 7,072,237 person-years of observation. Among HIV-infected patients, 208 were treated for ESRD during 53,196 person-years of follow-up (Table 2). Most of these cases occurred
among black patients (n =179; 86.1%). Concurrent diabetes was present in 27.9% of black versus 55.2% of white patients who had HIV and were treated for ESRD. A total of 9590 patients with diabetes were treated for ESRD during 2,057,353 person-years of follow-up. Most of these cases (n =6765;
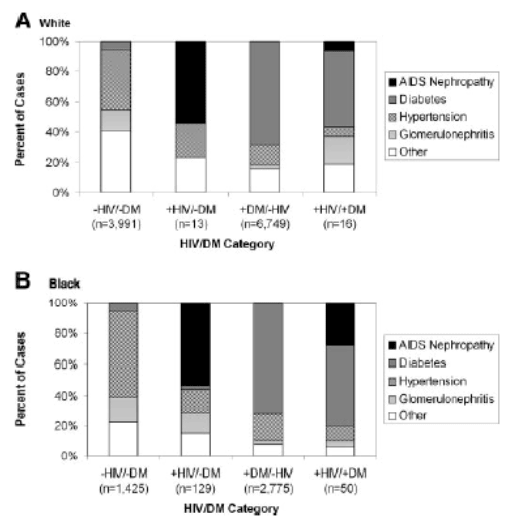
70.5%) occurred among white individuals. Among both white and black HIV-infected patients who developed ESRD during follow-up, AIDS nephropathy was the most common cause listed in the US Renal Data System (USRDS) PATIENTS file (Figure 1). When HIV and diabetes both were present, diabetes was the most frequently listed cause of ESRD.
Among black patients, the age- and sex-adjusted incidence of ESRD per 1000 person-years was higher among those with HIV (7.3; 95% confidence interval [CI] 6.0 to 8.6) compared with those without HIV or diabetes (2.3; 95% CI 2.2 to 2.5) but lower than for those with diabetes (8.9; 95% CI 8.5 to 9.3), as shown in Table 2.
Among white patients, the age- and sex-adjusted incidence of ESRD per 1000 person-years was similar between those with HIV (0.9; 95% CI 0.4 to 1.4) and those without HIV or diabetes (1.0; 95% CI 0.9 to 1.0) but much lower than those with diabetes (3.9; 95% CI 3.7 to 4.0). The unadjusted incidence of ESRD per 1000 person-years was 2.5 (95% CI 0.8 to 7.7) among black women with HIV and 2.6 (95% CI 2.2 to 3.1) among black women with diabetes.
In multivariate Cox proportional hazards analysis, both HIV (hazard ratio [HR] 4.56; 95% CI 3.44 to 6.05) and diabetes (HR 4.15; 95% CI 3.89 to 4.43) were strongly associated with ESRD among black patients when compared with the referent category (Table 2). Among white patients, diabetes was associated with an increased risk for ESRD (HR 2.18; 95% CI 2.07 to 2.29), but HIV was not (HR 0.76; 95% CI 0.45 to 1.30). When the risk for ESRD was compared between black and white HIV-infected individuals, the HR was 5.97 (95% CI 3.12 to 11-41).
This difference was attenuated when concomitant diabetes was present (HR 2.33; 95% CI 1.02 to 5.35). We did not find an interaction between HCV and HIV (P =0.122), but CKD modified the effect of HIV/diabetes status on the risk for ESRD among black patients. Among black patients with an eGFR <60 ml/min per 1.73 m2, HIV had an HR for ESRD of 3.59 (95% CI 2.57 to 5.04), whereas diabetes had an HR of 3.85 (95% CI 3.59 to 4.12). The risk for ESRD was magnified in black patients with an eGFR >60 ml/min per 1.73m2, where the HR was 10.46 (95% CI 7.36 to 14.86) for HIV and 7.14 (95% CI 6.24 to 8.16) for diabetes.
In the sensitivity analysis in which we included patients with unknown race, the adjusted HR for HIV in white patients ranged from 0.84 (95% CI 0.50 to 1.43) to 1.02 (95% CI 0.62 to 1.67), depending on whether unknown race was coded as black or white, respectively. Similarly, the adjusted HR for HIV among black patients ranged from 4.63 (95% CI 3.51 to 6.10) to 5.13 (95% CI 3.87 to 6.80). Adjusted HR for ESRD were not substantially different when we repeated the analysis using CKD categories defined by the higher of two eGFR levels at
least 3 mo after cohort entry. In the subgroup analysis of women, the adjusted HR for ESRD was 3.42 (95% CI 1.02 to 11.51) for black women with HIV and 3.98 (95% CI 2.60 to 6.11) for black women with diabetes.
DISCUSSION
In this large national cohort of veterans, the incidence of ESRD among black individuals with HIV was nearly an order of magnitude greater than that among white individuals with HIV.
Black individuals with HIV were as likely to develop ESRD as black individuals with diabetes and almost twice as likely to develop ESRD as white individuals with diabetes.
In contrast, the incidence of ESRD among white patients with HIV was similar to that among white patients without HIV or diabetes.
These findings demonstrate that race is a critical determinant of ESRD risk among HIV-infected patients and underscore the importance of HIV as a risk factor for ESRD among black individuals.
This study is the first to examine the association of HIV with the development of ESRD in a national cohort of patients. Our results confirm the widely recognized racial predilection of HIV-related renal diseases. In 1984, the first cases of HIV-associated nephropathy were reported, describing a form of FSGS associated with AIDS among 11 black patients in New York City.7 Subsequent case series of kidney disease among HIV- infected patients have also primarily been composed of young black men.8-11 Similarly, studies using ESRD registry data demonstrate that approximately 88% of all cases of ESRD in the HIV-infected
population occur among black individuals.6,12,13 Collectively, these reports support the racial disparity in ESRD-incidence among HIV-infected patients reported here.
Incidence rates of ESRD that were observed in our study among HIV-infected patients are comparable to the indirect estimates obtained by Eggers and Kimmel.6 By combining information on cause of ESRD in the USRDS with estimates of the size of the HIV-infected population from the Centers for
Disease Control and Prevention, these authors estimated the incidence of ESRD to be 3467 per 1,000,000 people with HIV, similar to the overall estimate of 3.6 (95% CI 3.2 to 4.2) per 1000 person-years in our cohort. Our findings are also consistent with previous work supporting an association between HIV and the development of non-dialysis-dependent CKD. In the HIV Epidemiology Research Study,4 after a mean observation time of 21 mo, 14% of HIV-infected women subsequently developed proteinuria or elevated creatinine compared with
only 4% in the HIV-uninfected group. In this study, black race was also identified as a risk factor for the development of CKD. The striking parity in the risk for ESRD observed between black individuals with HIV and diabetes may have implications for health policy and clinical practice. First, black individuals are the largest and fastest growing racial group with HIV in the United States. Half of all new HIV infections in the United States are among black individuals, who represent only 15% of its overall population.14 Our results demonstrate an excess risk for ESRD among black individuals with HIV and therefore lend support to projected increases in the size of the HIV-infected ESRD population.3
Furthermore, the World Health Organization reported that in 2006, 24.7 million people (63% of all people with HIV infection) were living with HIV in sub-Saharan Africa.15 Kidney biopsy studies have revealed that HIV-infected patients in Africa exhibit similar renal disease patterns compared with black individuals who live in the United States, France, and Brazil.16-20 Additional investigations are urgently needed to determine whether our results are applicable to the global burden of HIV disease. Second, these findings highlight the need for strategies to prevent and slow the progression of CKD among black individuals with HIV. Whereas evidence-based guidelines exist for the management of diabetic nephropathy, current recommendations
for the management of CKD in patients with HIV are largely opinion based.1 The substantial risk for ESRD in black individuals with HIV demonstrates the need for a credible, evidence-based approach to improve renal outcomes in this group, analogous to the state-of-the-art management of diabetic nephropathy.
Finally, although a disproportionate number of cases of ESRD in the United States occur among black individuals, there has been little advancement in our understanding of the underlying reasons for this racial susceptibility.21 Many potential mechanisms have been proposed, such as differences in SES, patient preferences, comorbid illnesses, environmental factors, and genetics.21-27 In the HIV-infected population, differences in HIV biology or the effectiveness and access to antiretroviral therapy may be additional contributors. The pronounced
racial differences in the risk for ESRD among HIV-infected individuals suggest that HIV-related kidney disease may serve as a valuable model for understanding the underlying mechanisms for this racial predilection.
Our study has the following limitations: (1) Misclassification of diabetes may have occurred, because we relied on administrative data to identify these patients; however, it is reassuring that the incidence of ESRD among cohort patients with
diabetes is consistent with previously published estimates in other cohorts.22,28 (2) Our results may not be generalizable to nonveteran populations and to women in particular. Nevertheless, it is unlikely that the race effect observed here would not be present in other populations. Results for women had wide CI, but point estimates for incidence and adjusted HR were qualitatively similar to our overall findings. (3) Although the wide range of data available allowed us to control for differences in level of renal function, comorbid conditions, and sociodemographic variables among patients who received outpatient care in the Department of Veterans Affairs (VA) health care system, we cannot rule out the possibility of residual confounding by information that could not be captured by ourdata sources. In particular, we were unable to adjust for proteinuria, BP measurements, the severity of comorbid conditions, access to treatment, or the intensity or regularity of follow-up care; however, the substantial magnitude of the association of HIV with ESRD among black patients, despite extensive multivariate adjustment, argues against unmeasured confounding as the sole explanation for our findings. (4) We were unable to determine whether differences in antiretroviral medication use contributed to the racial disparity in ESRD rates that were observed among HIV-infected individuals. Although
previous work in the VA29,30 found antiretroviral treatment rates to be no different between white and black patients, further study is needed to clarify the role of antiretroviral therapy in contributing to racial differences in renal outcomes
among HIV-infected individuals. (5) Since 2003, the VA adopted a new system of race reporting to conform with federal guidelines and in doing so established 95% agreement between observer-reported data with self-reported race.31 Although high-quality race data were available to us, race was unknown for many members of the cohort. Exclusion of these patients may have biased results; however, our sensitivity analysis demonstrates that our findings are robust to the potential effect of this misclassification. (6) Our study addresses the risk for ESRD only in black and white patients; therefore, other studies will be necessary to characterize rates of ESRD in other racial and ethnic groups.
In this national cohort of veterans, the incidence of ESRD among black patients with HIV was approximately 10 times that among white patients with HIV, almost twice that among white patients with diabetes, and similar to that among black patients with diabetes. These findings demonstrate that kidney disease in the setting of HIV infection disproportionately affects black individuals and emphasize the importance of defining strategies to understand, prevent, and treat CKD among black individuals who are infected with HIV.
CONCISE METHODS
Patients
We identified 2,352,584 veterans who were aged 18 to 100 yr and had at least one serum creatinine measured on an outpatient basis at a VA facility between October 1, 2000, and September 30, 2001. Patients entered the study at the time of their first creatinine measurement during this period. We excluded 11,125 patients who had already reached ESRD, defined as receipt of long-term dialysis or kidney transplant. A total of 300,038 (12.8%) patients with unknown race
and 25,530 (1.1%) patients of non-black, nonwhite race were excluded, leaving 2,015,891 patients for our analysis. Patients with unknown race were retained for a sensitivity analysis to test the robustness of primary study results to the effects of unknown race.
Data Sources
We used the following data sources to assemble the study cohort. Serum creatinine measurements associated with outpatient visits were obtained from the VA Decision Support System Laboratory Results file.32 We used the Immunology Case Registry (ICR) to identify all patients with HIV.33 The ICR is a national administrative database designed to monitor health care use among all HIV-infected patients who receive care in the VA system. Veterans with HIV are identified by local ICR coordinators who manually enter patients into a local
registry. We used the ICR, VA National Patient Care Database, Fee Basis files, Medicare Denominator File, and inpatient and outpatient Medicare claims to ascertain demographic and comorbidity information. 32 When available, we preferentially used race information from the Medicare Denominator File because of its superior reliability to VA race data.31,34 We determined the date of death for cohort members using the VA Beneficiary Identification and Records Locator
Subsystem.35-37 Area-level socioeconomic data were obtained by matching an individual's residential zip code at the time of cohort entry to year 2000 US Census zip code tabulation areas.38 Patients who were treated with long-term dialysis or kidney transplant were identified using the USRDS,39 a comprehensive national ESRD registry. We obtained information on the primary disease listed as the cause of ESRD using the USRDS PATIENTS file.
Outcomes
We measured time from cohort entry to onset of ESRD, defined as initiation of long-term dialysis or kidney transplantation through November 17, 2004. Patients who did not reach ESRD were censored at the time of death or the end of follow-up.
Primary Predictor Variables
The primary predictor variable for all analyses was whether the patient had HIV, diabetes, both conditions, or neither condition at the time of cohort entry. Patients were considered to have HIV when a diagnosis of HIV was confirmed before the time of cohort entry on the basis of previous treatment with antiretroviral therapy or the Centers for Disease Control and Prevention case definition.40 Diabetes status was designated using methods validated for VA administrative databases on the basis of information entered between January 1, 1999, and the time of cohort entry.41
Covariates
We adjusted for age, sex, level of renal function, and the following diagnosed conditions on the basis of International Classification of Diseases, Ninth Revision diagnostic codes and Current Procedural Terminology codes: hypertension, coronary artery disease (defined as a previous diagnosis of coronary artery disease, angina, or myocardial infarction or previous coronary artery bypass graft or angioplasty), congestive heart failure, peripheral arterial disease (defined as a previous diagnosis of peripheral arterial disease or previous lower extremity
amputation or revascularization procedure), chronic obstructive pulmonary disease, cerebrovascular disease (defined as previous stroke or transient ischemic attack), and HCV infection. For each patient, we estimated GFR using the abbreviated Modification of Diet in Renal Disease (MDRD) formula based on age, sex, race, and serum creatinine level at the time of cohort entry.42,43 Patients were classified according to level of eGFR as follows: eGFR >60 ml/min per 1.73 m2 (normal or mildly reduced), eGFR 30 to 59 ml/min per 1.73
m2 (moderately reduced), eGFR 15 to 29 ml/min per 1.73 m2 (severe reduced), and eGFR <15 ml/min per 1.73 m2 and not on dialysis (renal failure).
Analyses were also adjusted for the following zip code-level socioeconomic
characteristics derived from the US Census: Median household income; median value of housing units; percentage of residents who are older than 25 yr and high school graduates; percentage of residents who are older than 25 yr and have a bachelor's degree; percentage of residents in executive, managerial, or professional specialty occupations; percentage of families below the poverty level; and percentage of housing units occupied by the owner. Using a technique
reported previously for census blocks,44 we calculated a z score for each zip code by subtracting the mean value for the variable and dividing by the SD. Overall summary scores for SES were derived for each patient by summing z scores for each socioeconomic variable. Patients were then divided into tertiles according to summary socioeconomic scores. Socioeconomic information was not available because of missing zip code information for 144 (0.01%) patients or because zip codes could not be linked to a zip code tabulation area in the US Census (63,723 [3.2%] patients). For patients with missing information on zip code SES, we included a missing indicator variable for SES in multivariate analysis.
Statistical Analyses
Age- and sex-adjusted incidence rates for ESRD were obtained using a parametric survival-time model fitted to the exponential distribution and standardized to the median age. We used Cox proportional hazard analysis to examine the association of predictor variables with time to ESRD. Estimates were adjusted for age, sex, baseline comorbidities, level of renal function, and SES. A robust sandwich variance estimator was used to account for clustering within zip code of residence.
We tested for interactions between the HIV/diabetes predictor variable and race, HCV infection, and the presence of CKD at baseline. The analysis of rates of ESRD was stratified by white/black race to accommodate a significant race interaction (P <0.001).
We performed a sensitivity analysis to assess the potential impact of unknown race on our results. Analyses were replicated after coding all individuals with unknown race as white. Likewise, we also obtained results after coding all of these individuals as black. To assess the impact of misclassification of level of renal function on the basis of a single creatinine measurement at the time of cohort entry, we also conducted a subgroup analysis among patients with a repeat serum creatinine measurement at least 3moafter cohort entry, in which level of renal function was classified on the basis of the higher of two eGFR measurements. We also repeated the primary analyses among women. The proportional hazards assumption was checked using the Schoenfeld test and by graphically comparing stratified log[ log(survivor function)] versus time curves. Model assumptions were checked using bootstrap methods. Analyses were conducted using Stata 9.0 (StataCorp, College Station, TX). This study was approved by the institutional review board at the University of California, San Francisco, and the Research Committee at the VA San Francisco.
|
|
| |
| |
|
|
|