| |
Racial Differences in Virologic Failure Associated With Adherence and Quality of Life on Efavirenz-Containing Regimens for Initial HIV Therapy: Results of ACTG A5095
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes:Volume 46(5)15 December 2007pp 547-554
Schackman, Bruce R PhD*; Ribaudo, Heather J PhD; Krambrink, Amy MS; Hughes, Valery FNP; Kuritzkes, Daniel R MD; Gulick, Roy M MD, MPH
From the *Department of Public Health, Weill Medical College, New York, NY; Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, MA; Division of International Medicine and Infectious Diseases, Weill Medical College, New York, NY; and the Section of Retroviral Therapeutics, Brigham and Women's Hospital and Division of AIDS, Harvard Medical School, Boston, MA.
"...Patients on efavirenz-based regimens in the ACTG A5095 study did well, with at least 80% having HIV-1 RNA suppressed to <50 copies/mL through 3 years (144 weeks) of follow-up.32 Self-reported adherence over time was also quite good; at each evaluation, on average, 84% of subjects reported missing no doses in the past 4 days and 67% of subjects reported missing no doses in the past month. Nevertheless, patients who reported missing doses were at a significantly higher risk of virologic failure....
...A significant interaction between race and recent (time-dependent) adherence in their association with virologic failure was observed in Cox proportional hazard models adjusted for baseline covariates for the metrics missed past weekend (P = 0.05), missed past month (P < 0.01), and ever missed (P = 0.04). Although not statistically significant (P = 0.17), a similar trend was observed for the nonadherence during the past 4 days metric. Figure 2 demonstrates the nature of this interaction: nonadherent whites had an estimated probability of maintaining a virologic response over time that was similar to adherent whites and adherent blacks, but nonadherent blacks had a lower probability of maintaining a virologic response; this was observed consistently over time and across all four metrics....
....an interaction between race and adherence in predicting virologic failure was supported using several different adherence metrics. The reasons for this association remain unclear but may be related to genetic differences between blacks and whites in efavirenz metabolism or to undetected differences in adherence patterns attributable to other factors. Most blacks in this study did well on efavirenz-based regimens. Our findings indicate that clinicians should monitor adherence to efavirenz particularly closely among black patients, however, and be ready to provide referrals for adherence counseling, toxicity management, and/or social support when indicated; the incremental cost of these services is low compared with the cost of nonadherence"
Abstract
Background: Blacks had higher rates of virologic failure than whites on efavirenz-containing regimens in the AIDS Clinical Trials Group (ACTG) A5095 study; preliminary analyses also suggested an association with adherence. We rigorously examined associations over time among race, virologic failure, 4 self-reported adherence metrics, and quality of life (QOL).
Methods: ACTG A5095 was a double-blind placebo-controlled study of treatment-naive HIV-positive patients randomized to zidovudine/lamivudine/abacavir versus zidovudine/lamivudine plus efavirenz versus zidovudine/lamivudine/abacavir plus efavirenz. Virologic failure was defined as confirmed HIV-1 RNA ≥200 copies/mL at ≥16 weeks on study. The zidovudine/lamivudine/abacavir arm was discontinued early because of virologic inferiority. We examined virologic failure differences for efavirenz-containing arms according to missing 0 (adherent) versus at least 1 dose (nonadherent) during the past 4 days, alternative self-reported adherence metrics, and QOL. Analyses used the Fisher exact, log rank tests, and Cox proportional hazards models.
Results: The study population included white (n = 299), black (n = 260), and Hispanic (n = 156) patients with ≥1 adherence evaluation.
Virologic failure was associated with week 12 nonadherence during the past 4 days for blacks (53% nonadherent failed vs. 25% adherent; P < 0.001) but not for whites (20% nonadherent failed vs. 20% adherent; P = 0.91).
After adjustment for baseline covariates and treatment, there was a significant interaction between race and week 12 adherence (P = 0.02). In time-dependent Cox models using self-reports over time to reflect recent adherence, there was a significantly higher failure risk for nonadherent subjects (hazard ratio [HR] = 2.07; P < 0.001).
Significant race-adherence interactions were seen in additional models of adherence: missing at least 1 medication dose ever (P = 0.04), past month (P < 0.01), or past weekend (P = 0.05). Lower QOL was significantly associated with virologic failure (P < 0.001); there was no evidence of an interaction between QOL and race (P = 0.39) or adherence (P = 0.51) in predicting virologic failure.
Conclusions: There was a greater effect of nonadherence on virologic failure in blacks given efavirenz-containing regimens than in whites. Self-reported adherence and QOL are independent predictors of virologic failure.
Strict adherence to antiretroviral medications has been shown to improve HIV virologic and clinical outcomes significantly.1-4 The association between nonadherence and virologic failure varies, however, depending on drug class, target population, and adherence metric. Adherence rates >95% resulted in maximum virologic suppression in studies in which subjects were receiving unboosted protease inhibitor-based regimens.5,6 In contrast, studies of regimens using nonnucleoside reverse transcriptase inhibitors have suggested threshold adherence rates of 75% or even lower based on patient self-report, electronic medication monitoring, or unannounced pill counts.7,8
Self-reported adherence is a reliable predictor of virologic response.9-11 Strategies to improve the sensitivity of this assessment include using adherence data collected over several time intervals12 and using multiple self-reported adherence question responses.13 Sociodemographic factors are inconsistently associated with adherence to antiretroviral therapy,14 but several studies indicate that blacks may have poorer adherence than whites.15-19 Few contemporary studies have explored race as an independent predictor of virologic response. In one urban cohort study, black race was associated with a decrease in virologic response but not in survival.20 No association was observed between race and virologic failure in the Women's Interagency HIV Study after adjusting for discontinuation of antiretroviral therapy, depression, and baseline factors.21 Hartzell et al22 found, however, that black race and mental health diagnosis were independent predictors of virologic failure after 24 months in a military cohort.
Quality of life (QOL) is another self-reported assessment that is often collected in HIV clinical trials. We hypothesized that patients who self-reported poor QOL might be less likely to adhere to antiretroviral therapy if their QOL was adversely affected by depression or by symptoms that they attributed to antiretroviral medication. Therefore, we concluded that this measure should also be explored when investigating racial differences in virologic response. There is mixed evidence about the association between viral load and QOL in cross-sectional studies,23,24 but improvements in QOL have been reported for patients receiving antiretroviral treatment and achieving a reduction in HIV-1 viral load in clinical trials25,26 and cohort studies.27,28 A simple rating scale assessment has been used to obtain a summary evaluation of self-reported QOL in clinical studies and in economic evaluations of HIV treatment.24,29,30 The utility of QOL measured in this fashion as a predictor of virologic response and differences in how the scale predicts virologic response by race have not been evaluated, however. In a cross-sectional AIDS Clinical Trials Group (ACTG) cohort study, nonwhites self-reported higher rating scale assessments of QOL than whites after adjustment for disease severity and other covariates, but the effect size was small.24
ACTG A5095 was a randomized, double-blind, placebo-controlled study of 3 protease inhibitor-sparing regimens in antiretroviral-naive subjects. The triple-nucleoside arm was discontinued early because of virologic inferiority.31 Subjects in the 3- and 4-drug efavirenz-based regimen arms continued blinded follow-up through a median of 3 years. There were no significant differences in virologic response between the 3-drug and 4-drug regimens, but there was an increased risk for virologic failure in non-Hispanic black patients compared with non-Hispanic white patients.32 We also reported a significant interaction between race and self-reported adherence at week 12 in the ACTG A5095 study population. This suggested that blacks who reported missing at least 1 dose of their efavirenz-based regimen over the prior 4 days had a significantly shorter time to virologic failure than similarly nonadherent whites. In this study, we examine the robustness of this result by comparing 4 definitions of adherence and by varying the timing of the adherence assessment beyond week 12. In addition, we evaluate QOL as an independent predictor of virologic failure and its potential interactions with race and adherence as predictors of virologic failure.
METHODS
ACTG A5095 Study Design
ACTG A5095 was designed as a randomized, double-blind, placebo-controlled study of 3 initial antiretroviral regimens for treatment of HIV-1 infection: zidovudine/lamivudine/abacavir (triple-nucleoside regimen), zidovudine/lamivudine plus efavirenz (3-drug regimen), or zidovudine/lamivudine/abacavir plus efavirenz (4-drug regimen). Adult patients with HIV-1 infection who had not taken prior antiretroviral therapy with a plasma HIV-1 RNA level ≥400 copies/mL were eligible. The primary efficacy endpoint, time to virologic failure, was defined as the time to the first of 2 successive HIV-1 RNA levels ≥200 copies/mL at or after week 16. This endpoint was selected to avoid false-positive results attributable to intermittent viremia and to reflect clinical practice.33
Initially the study regimen consisted of 7 pills (including matching placebos), divided twice daily; the study regimen was reduced to 5 pills, divided twice daily, when 600-mg efavirenz tablets became available. For study drug toxicity considered treatment-limiting by the site investigator, patients could substitute stavudine for zidovudine, didanosine for abacavir, and/or nevirapine for efavirenz and were still considered to be on their initial regimen.
In February 2003, the National Institute of Allergy and Infectious Diseases Data Safety Monitoring Board (DSMB) concluded that the triple-nucleoside regimen was virologically inferior to each of the 2 efavirenz-containing regimens, meeting protocol-specified stopping criteria. The DSMB recommended that the triple-nucleoside group be discontinued and unblinded and that data be released comparing results from this group with pooled data from the other 2 groups.31 The 2 groups taking efavirenz-containing regimens completed planned follow-up blinded to 4 versus 3 drugs (abacavir or not).
With a median 144 weeks of follow-up, time to virologic failure was not significantly different between the 4-drug regimen and the 3-drug regimen; this result remained consistent after adjusting for gender, age, race/ethnicity, hepatitis B/C virus status, baseline HIV-1 RNA, and baseline CD4.32 The data presented here are for complete follow-up of the 2 efavirenz-containing regimens.
Assessments of Adherence and Quality of Life
An adherence questionnaire34 was self-administered by the subject, completed by the study coordinator during an interview with the subject, or completed using both methods. Responses were collected at baseline (for QOL only); at weeks 4, 12, and 24; and then every 24 weeks or, if applicable, at the time of confirmed virologic failure or permanent study drug discontinuation. Each of these visits also included clinical assessments and laboratory testing, including plasma HIV-1 RNA. The questionnaire includes a day-by-day assessment of doses of each prescribed antiretroviral drug missed in the past 4 days and questions about the last time that medications were skipped and about adherence during the previous weekend.
Based on these questions, we derived 4 adherence variables indicating whether or not the subject reported perfect adherence during different recall periods (see Table 1 for definitions): past 4 days, past weekend, past month, and ever missed. These binary metrics were used to define adherent and nonadherent subgroups in the analyses. An additional quality-of-life (QOL) question asks subjects to rate their overall health on a 0-to-100 scale in response to the question How would you rate your current state of health from 0 to 100? where 0 represents death or worst possible health and 100 represents perfect or best possible health.
Statistical Methods
We examined whether there was an association between adherence or QOL and virologic failure at different time points and differences in these associations between blacks and whites. Based on preliminary analyses using the past 4 days adherence metric, we determined that using data on adherence evaluated at week 12 and conducting time-dependent analyses allowed us to examine the greatest number of virologic failure endpoints with the closest proximity of time between adherence and failure. Time-updated adherence covariates were used to examine the effect of recent adherence. Because adherence was only collected at the specified time points, at each event time, recent adherence for each subject was defined based on the subject's most recent adherence evaluation within the previous 24 weeks. If an adherence evaluation was not completed within that time period, adherence data were considered missed.
Log-rank tests were used to compare the failure time distributions according to adherence versus nonadherence over the recall periods measured at week 12. An extension of the method of Kaplan-Meier was used to estimate the distribution of virologic failure according to recent adherence versus nonadherence.35 Cox proportional hazards models were used to estimate the relative hazard of failure according to week 12 adherence and recent adherence. Subjects missing recent adherence information at a given failure time were excluded at that time but could still contribute to the risk sets at other failure times. Subjects with no adherence evaluations (n = 35) were excluded; all had no more than 4 weeks of follow-up on treatment, and their baseline characteristics were similar to those of other subjects. Univariate analyses were performed, and additional analyses were adjusted for gender, race/ethnicity, age, baseline HIV-1 RNA level, baseline CD4 cell count, hepatitis B/C virus status, and treatment (3- vs. 4-drug regimen).
Following a similar analysis plan to the adherence analyses, the associations between virologic failure and QOL were examined at week 12 and using QOL as a time-updated covariate (to reflect recent QOL). Again, univariate analyses were performed, and additional analyses were adjusted for adherence, race/ethnicity, and the other potential baseline confounders. Linearity of the effect of the QOL responses on a continuous scale was assessed by dividing the data into subgroup quintiles of response and then plotting subgroup-specific parameter estimates against the median response for each subgroup. Because the linearity assumption was questionable, 3 QOL score categories were subsequently analyzed: <75, 75 to 89, and ≥90. These categories were chosen to be clinically meaningful based on prior reports24 and to provide constant effects within each subgroup (based on examination of the quintile-specific effects). Because self-reported QOL measurement may have greater clinical relevance when it occurs closely in time before virologic failure has been observed, additional sensitivity analyses were conducted with recent QOL defined as QOL reported during the study visit strictly before the visit where the first measurement of virologic failure took place.
RESULTS
Study Population
Of the 765 patients taking efavirenz-containing regimens, 730 completed at least 1 adherence evaluation. The race/ethnicity of these patients was self-reported as non-Hispanic white (white; n = 299); non-Hispanic black (black; n = 260); Hispanic of any race (n = 156); and Asian/Pacific Islander, American Indian/Alaskan Native, or other (n = 15). Patients in the last group were excluded from all analyses taking into account race/ethnicity because of the small sample size. Of the remaining 715 patients, data were available on 84% at 12 weeks and on 76% at 48 weeks.
Adherence and Virologic Failure
Over the course of the study, the average proportion of subjects reporting no doses missed during the past 4 days was 84% (range by evaluation week: 82% to 92%), the proportion reporting no doses missed during the past weekend was 90% (89% to 93%), and the proportion reporting no doses missed during past month was 67% (65% to 70%); on average, at each evaluation, 48% of subjects reported never missing a dose (39% to 66%). Responses to the different adherence metrics were consistent. For instance, 87% of subjects reporting doses missed by the past 4 days metric also reported doses missed in the past month. For the 715 subjects with at least 1 evaluation of adherence, 354 (50%) always reported being adherent (no doses missed) using the past 4 days metric, 150 (21%) reported being adherent at 75% to 99% of their evaluations, 124 (17%) reported being adherent at 50% to 75% of their evaluations, and 87 (12%) reported being adherent at <50% of their evaluations (including 3% who reported being nonadherent at all evaluations). Repeated self-reports of being adherent occurred less frequently for the adherence metrics with longer recall periods; for instance, only 19% of subjects reported never missing a dose at all evaluations (see Table 1).
Race/Ethnicity and Adherence
We previously reported that using the missed past 4 days metric, there were significantly higher proportions of nonadherent blacks versus whites at week 4 (20% vs. 11%) and week 12 (22% vs. 12%) (P = 0.01 for both weeks) but not at subsequent weeks.32 Virologic failure was associated with week 12 nonadherence during the past 4 days for blacks (estimated cumulative probability of failure by week 144 was 53% for nonadherent vs. 25% for adherent; P < 0.001) but not for whites (20% for nonadherent vs. 20% for adherent; P = 0.91).
Similar results indicating an interaction between race and adherence in their association with virologic failure were seen using alternative week 12 adherence metrics (Table 2). In Cox models, a significant interaction between race and week 12 adherence, indicating a greater impact of week 12 nonadherence in blacks compared with whites, remained after adjusting for baseline covariates using all metrics: missed during the past 4 days (P = 0.02), missed during the past weekend (P = 0.02), missed during the past month (P = 0.02), and ever missed (P = 0.09).
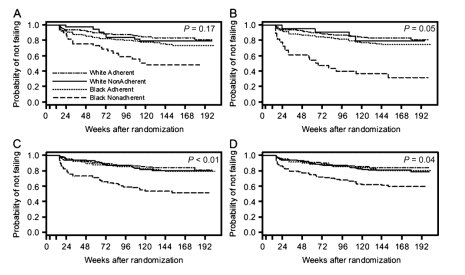
A significant interaction between race and recent (time-dependent) adherence in their association with virologic failure was observed in Cox proportional hazard models adjusted for baseline covariates for the metrics missed past weekend (P = 0.05), missed past month (P < 0.01), and ever missed (P = 0.04). Although not statistically significant (P = 0.17), a similar trend was observed for the nonadherence during the past 4 days metric. Figure 2 demonstrates the nature of this interaction: nonadherent whites had an estimated probability of maintaining a virologic response over time that was similar to adherent whites and adherent blacks, but nonadherent blacks had a lower probability of maintaining a virologic response; this was observed consistently over time and across all four metrics.
FIGURE 2. Time to virologic failure by recent adherence for non-Hispanic whites and non-Hispanic blacks for each of the 4 adherence metrics: missed during past 4 days (A), missed during past weekend (B), missed during past month (C), and ever missed (D). P values are from Cox proportional hazard models testing against the null hypothesis of an equivalent effect of recent adherence within race (black vs. white).
Quality of Life and Virologic Failure
Mean (SD) QOL scores increased from 73.0 (19.5) at baseline to 78.3 (16.9) at week 12, 80.7 (15.9) at week 48, 82.4 (15.3) at week 96, and 83.3 (14.4) at week 144. The distributions of QOL scores were similar for subgroups defined by 4-day adherence, race/ethnicity, age, gender, and hepatitis B/C virus status.
A Cox proportional hazard model with week 12 QOL scores adjusted for week 12 adherence (using the missed past 4 days metric) showed an increased risk of virologic failure for subjects with QOL scores <75 (HR = 2.18, 95% CI: 1.45 to 3.28; P < 0.001) and subjects with QOL scores from 75 to 89 (HR = 1.61, 95% CI: 1.06 to 2.45; P = 0.03) compared with subjects with QOL scores ≥90. In evaluating the association between QOL and virologic failure, there were no significant interactions between week 12 QOL categories and week 12 adherence in the past 4 days (P = 0.64), race/ethnicity (P = 0.53), hepatitis C status (P = 0.65), or age (P = 0.16).
Figure 3 shows an extended Kaplan-Meier plot of recent QOL as a time-dependent covariate. In a Cox model with recent QOL and recent 4-day adherence as time-dependent variables, subjects with recent QOL scores of <75 and 75 to 89 had more than twice the risk of virologic failure of subjects with recent QOL scores ≥90 (HR = 2.58, 95% CI: 1.67 to 3.99; HR = 2.12, 95% CI: 1.38 to 3.27, respectively). In evaluating the association between recent QOL and virologic failure, there were no significant interactions between recent QOL and recent adherence or other baseline covariates, including race. Results were comparable when recent QOL was defined as QOL self-reported strictly during the previous study week visit.
DISCUSSION
Patients on efavirenz-based regimens in the ACTG A5095 study did well, with at least 80% having HIV-1 RNA suppressed to <50 copies/mL through 3 years (144 weeks) of follow-up.32 Self-reported adherence over time was also quite good; at each evaluation, on average, 84% of subjects reported missing no doses in the past 4 days and 67% of subjects reported missing no doses in the past month. Nevertheless, patients who reported missing doses were at a significantly higher risk of virologic failure. Importantly, we observed this association even when using a strict definition of adherence requiring consistent responses (ie, self-reporting missing no doses in the past 4 days and never missing medications over a longer recall period).
A notable observation in the ACTG A5095 study was that for patients on efavirenz-based regimens, there was a significantly shorter time to virologic failure in blacks compared with whites. Racial/ethnic differences in efavirenz-based treatment responses are critical to understand, because these drugs are recommended as first-line therapy in diverse populations worldwide.36 In this study, we explored the relation between adherence, race, and virologic failure using 4 different adherence metrics and time-dependent analyses of recent adherence. We confirmed our previous finding of a significant interaction between race and adherence in which nonadherent blacks taking efavirenz-based regimens had a shorter time to virologic failure than nonadherent whites using 4-day recall assessed at week 12 of the study. We previously reported that no interaction between race and adherence was seen in patients taking the triple-nucleoside regimen in this trial, although there was shorter follow-up time on these patients because of the early closure of this arm (median follow-up of 32 weeks).32
The interaction between race and adherence in predicting virologic failure that we observed could be attributable to racial differences in adherence patterns that we were unable to assess using the self-reported metrics in this study. Although there are no published data to support these suppositions, one could hypothesize that blacks are more likely to underreport missed doses than whites, perhaps because of differential social desirability effects by race; therefore, a report of 1 missed dose among blacks may represent a lower actual level of adherence compared with whites. Blacks could also be more likely to report a self-discontinuation or interruption of treatment as a missed dose. Poorer adherence in blacks compared with whites has been reported in studies of cardiac and diabetes medications using prescription refill data.37,38 A significant proportion of blacks have been reported to hold conspiracy beliefs about HIV/AIDS and negative attitudes toward antiretroviral medication,39,40 although blacks are less likely than whites to report experiencing discrimination from HIV health care providers.41
An alternative explanation is that the association between adherence and virologic failure is different on average in blacks versus whites. For example, this difference could be attributable to a genetic polymorphism more common in blacks that results in higher efavirenz levels, more drug toxicity, more drug discontinuation, and the development of drug resistance when medication is self-discontinued. The presence of the T/T genetic polymorphism at position 516 that codes for the hepatic enzyme responsible for efavirenz metabolism, CYP2B6, occurs more commonly in blacks. Among ACTG A5095 study patients enrolled in the smaller substudy A5097s, there was an association between this polymorphism and significantly higher efavirenz plasma exposures and early central nervous system symptoms, although no differences were observed in treatment discontinuation, tolerability, or virologic response rates.42 Predicted plasma efavirenz exposure after treatment discontinuation was also prolonged at high levels for patients with the polymorphism (regardless of race),43 and this, too, could lead to prolonged drug levels that increase the risk of drug resistance.
We previously reported that no significant interaction between race and adherence was observed using self-reported adherence over 4 days at week 4. It is difficult to interpret associations between virologic failure and week 4 self-reported adherence in this trial, because the definition of virologic failure used precluded failure before week 16. Similarly, associations between adherence at weeks 24 and 48 and virologic failure were not examined in detail, because these evaluations are only relevant for subjects with an extended period of successful virologic suppression. In contrast, the time-dependent analyses provide the strongest evidence of a proximate association between nonadherence and failure.
This is the first study to assess a simple rating scale evaluation of QOL as a predictor of virologic failure in patients on antiretroviral therapy. Although we expected to find that self-reported QOL would interact with adherence, we found that it was an independent predictor of virologic failure after controlling for self-reported adherence and race. Our results were consistent when we limited the analysis to QOL scores obtained at the study visit before virologic failure was observed. This suggests that self-reported QOL should be further evaluated for its clinical utility in identifying patients at risk of virologic failure and enhancing current self-reported adherence assessments, especially because it seems to perform similarly among blacks and whites.
Poorer self-reported QOL may be an indication of depression, other mental health diagnoses, or related psychosocial findings such as hopelessness. These conditions have been identified as independent predictors (after controlling for adherence and race) of virologic failure, adverse CD4 cell count and viral load changes, and clinical progression.22,44,45 Alternatively, the QOL measure may be associated with some aspect of adherence behavior that is not adequately assessed by the adherence metrics we used, such as treatment interruptions, that could be identified using other adherence questions.
The findings on QOL as a predictor of virologic failure need to be validated by further studies in diverse HIV populations. It should be noted that the number of subjects with missing QOL evaluations increased over time; therefore, the increase in QOL scores observed during the study could also be attributed to early dropouts of subjects with lower QOL scores.
This study has several limitations. We examined only patients enrolled in the ACTG A5095 study who were assigned to efavirenz-containing regimens, and patients with poor adherence may have been less likely to be referred by clinicians for study enrollment. As in other clinical trials, patients enrolled in this study self-reported better adherence than is observed generally in clinical practice, and it is possible that different racial patterns of adherence might be observed in other settings. The uniqueness of our findings to these regimens cannot be clearly assessed because of the early closure of the triple-nucleoside study arm.
In summary, in the ACTG A5095 study, efavirenz-based antiretroviral regimens for initial HIV treatment were generally well tolerated and most patients achieved durable virologic suppression. Although patients on efavirenz-based regimens with moderate levels of adherence had a good chance of virologic suppression, the probability of continued virologic suppression was significantly improved for patients with maximum adherence. Moreover, an interaction between race and adherence in predicting virologic failure was supported using several different adherence metrics. The reasons for this association remain unclear but may be related to genetic differences between blacks and whites in efavirenz metabolism or to undetected differences in adherence patterns attributable to other factors. Most blacks in this study did well on efavirenz-based regimens. Our findings indicate that clinicians should monitor adherence to efavirenz particularly closely among black patients, however, and be ready to provide referrals for adherence counseling, toxicity management, and/or social support when indicated; the incremental cost of these services is low compared with the cost of nonadherence.46 We also found that a simple question measuring self-reported QOL may be a clinically useful tool for identifying patients at risk of virologic failure on this regimen. Further study of these findings should have important benefits in optimizing clinical outcomes for the large numbers of patients receiving efavirenz-based initial HIV therapy worldwide.
|
|
| |
| |
|
|
|