| |
Thiazolidinediones and Cardiovascular Outcomes in Older Patients With Diabetes
|
| |
| |
JAMA Dec 12, 2007
Lorraine L. Lipscombe, MD, MSc; Tara Gomes, MHSc; Linda E. Levesque, BScPhm, MSc; Janet E. Hux, MD, MSc; David N. Juurlink, BPhm, MD, PhD; David A. Alter, MD, PhD
JAMA. 2007;298(22):2634-2643.
"....In summary, in this population-based study of older community-dwelling patients with diabetes, TZD treatment was associated with a significant increase in the risks of CHF, AMI, and death compared with other oral hypoglycemic agent treatments. These findings provide evidence from a real-world setting and support data from clinical trials that the harms of TZDs may outweigh their benefits, even in patients without obvious baseline cardiovascular disease. Further studies are needed to better quantify the risk-benefit trade-offs associated with TZD therapy and to explore whether the hazards associated with these agents are specific to rosiglitazone. In the interim, treatment decisions must remain individualized, with clinicians weighing the potential benefits and harms of TZD treatment, especially among high-risk elderly populations."
ABSTRACT
Context Thiazolidinediones (TZDs), used to treat type 2 diabetes, are associated with an excess risk of congestive heart failure and possibly acute myocardial infarction. However, the association between TZD use and cardiovascular events has not been adequately evaluated on a population level.
Objective To explore the association between TZD therapy and congestive heart failure, acute myocardial infarction, and mortality compared with treatment with other oral hypoglycemic agents.
Design, Setting, and Patients Nested case-control analysis of a retrospective cohort study using health care databases in Ontario. We included diabetes patients aged 66 years or older treated with at least 1 oral hypoglycemic agent between 2002 and 2005 (N = 159 026) and followed them up until March 31, 2006.
Main Outcome Measures The primary outcome consisted of an emergency department visit or hospitalization for congestive heart failure; secondary outcomes were an emergency department visit or hospitalization for acute myocardial infarction and all-cause mortality. The risks of these events were compared between persons treated with TZDs (rosiglitazone and pioglitazone) and other oral hypoglycemic agent combinations, after matching and adjusting for prognostic factors.
Results During a median follow-up of 3.8 years, 12 491 patients (7.9%) had a hospital visit for congestive heart failure, 12 578 (7.9%) had a visit for acute myocardial infarction, and 30 265 (19%) died. Current treatment with TZD monotherapy was associated with a significantly increased risk of congestive heart failure (78 cases; adjusted rate ratio [RR], 1.60; 95% confidence interval [CI], 1.21-2.10; P < .001), acute myocardial infarction (65 cases; RR, 1.40; 95% CI, 1.05-1.86; P = .02), and death (102 cases; RR, 1.29; 95% CI, 1.02-1.62; P = .03) compared with other oral hypoglycemic agent combination therapies (3478 congestive heart failure cases, 3695 acute myocardial infarction cases, and 5529 deaths). The increased risk of congestive heart failure, acute myocardial infarction, and mortality associated with TZD use appeared limited to rosiglitazone.
Conclusion In this population-based study of older patients with diabetes, TZD treatment, primarily with rosiglitazone, was associated with an increased risk of congestive heart failure, acute myocardial infarction, and mortality when compared with other combination oral hypoglycemic agent treatments.
COMMENT
Using population-based health care data, we found that TZD treatment was associated with a significant increase in the risks of CHF, AMI, and all-cause mortality among older persons with diabetes compared with other oral diabetes treatment. Moreover, the incremental risks associated with TZDs persisted even after adjustment for a number of important prognostic factors and were independent of baseline cardiovascular risk or diabetes duration. Treatment with TZDs was also associated with a higher risk of CHF and death regardless of whether it was used as monotherapy or in combination with other oral agents, further enhancing the argument for a causal relationship with these outcomes. Our findings argue against current labeling of TZDs that warns against use only in persons at high risk of CHF,20 as we did not identify any subgroup of older diabetes patients who may be protected from the adverse effects of TZDs.
This is to our knowledge the first study to evaluate TZD-related outcomes among an entire population of older patients with diabetes. While there have been previous attempts to evaluate the association between TZDs and CHF in real-world settings, prior studies were either small in sample size, composed of lower-risk diabetes populations, or insufficiently adjusted for case-mix.13, 15, 19, 41 In contrast, our study rigorously matched cases and controls and, thereafter, used additional adjustments for key prognostic indicators. Our large sample size also permitted a careful comparison of TZD use with other hypoglycemic regimens and subgroup analyses by baseline cardiovascular risk and diabetes duration. Also, with the exception of modest co-payment dispensing fees, which apply to all drugs, TZDs were provided free of charge to this patient population, minimizing the potential confounding effects of selection bias due to affordability factors.
Ours is also the first study to our knowledge to document an increase in mortality among TZD users. While 1 meta-analysis of clinical trials found a trend toward higher deaths with rosiglitazone,21 other meta-analyses either found no effect of TZDs17 or rosiglitazone18 on deaths or a possible protective effect of pioglitazone on mortality despite an increased CHF risk.8 One observational study documented a lower risk of death with TZDs among diabetes patients with CHF compared with no treatment with either TZDs or metformin.42 Although we could not determine cause of death in our study, the fact that cardiovascular events were also increased with TZDs suggests a possible cardiovascular etiology in this older, high-risk population.
The association between TZD treatment and cardiovascular events appeared to be limited to rosiglitazone. Our findings are consistent with recent studies that showed an increase in AMI risk18, 21 and possibly death21 with rosiglitazone. However, whereas others have documented a protective association between pioglitazone and risk of AMI8, 10, 43 and death,8, 10 we found no association between pioglitazone and these outcomes. Moreover, in contrast to clinical trial data, which suggest that both pioglitazone and rosiglitazone are associated with an increased risk of CHF,8, 10, 17, 19 we observed this association only with rosiglitazone. Our study may have been underpowered to detect adverse effects associated with pioglitazone because of the relatively small number of persons prescribed pioglitazone monotherapy. The reason for these observed differences is unclear, and larger studies are needed to better determine the relative effect of each agent on cardiovascular outcomes.
During the recent US Food and Drug Administration hearing regarding the safety of rosiglitazone, the advisory panel concluded that the available data are thus far inconclusive to warrant withdrawing rosiglitazone from the market.22 Their review of the evidence found that the association between rosiglitazone and adverse cardiac effects was based mainly on clinical trials not specifically designed to examine cardiac events or small observational studies of short duration.22, 44 Our large, well-designed population-based study provides more convincing evidence that rosiglitazone is associated with an increased risk of cardiac events and deaths among elderly patients with diabetes. Moreover, notwithstanding our study design, the magnitude of association between TZDs and adverse outcomes was consistent with risks reported elsewhere.10, 13-16,18-19,21 For example, both rosiglitazone meta-analyses reported an approximate 40% increased risk of AMI compared with placebo,18, 21 which is similar to the risk increase in our study. Such findings reaffirm the notion that relative treatment effects associated with most evidence-based pharmacotherapies are homogeneous across subgroups.45
The high baseline risk of cardiovascular events in older patients with diabetes underscores the potential adverse effects associated with TZDs in this population. Our results suggest that the numbers needed to harm over 4 years were approximately 26 for AMI, 34 for CHF, and 22 for death. The magnitude of harm observed in our study may therefore be sufficient to outweigh any potential benefits associated with TZDs in an older, higher-risk population. Moreover, only pioglitazone has been shown to reduce the incidence of major cardiovascular events,8, 10 whereas evidence for benefit of rosiglitazone is derived primarily from studies of glycemic durability and surrogate cardiovascular markers.4, 9, 14 While variations in the baseline risks of populations make the balance of risks and benefits of these drugs challenging, our results provide an important benchmark of absolute benefits that would need to be attained to overcome the hazards associated with TZDs.
Some limitations of our study merit emphasis. First, the use of administrative data confers a potential for misclassification of some exposures, covariates, and outcomes. This was minimized by using validated end points of CHF and AMI hospital visits as outcomes. Second, although we carefully matched cases and controls on important risk factors and adjusted for a number of potential confounders, we cannot completely exclude a selection bias in our findings. Third, we were not able to identify TZD use paid for by patients within our cohort. However, this potential bias would attenuate our effect sizes if some TZD users were misclassified as nonusers. Fourth, our TZD-treated population may represent an older and more select population of patients with more advanced diabetes because under Ontario Drug Benefit reimbursement criteria, most of these patients will have failed or had a contraindication to other drugs. Even though this may limit the generalizability of our findings, we found a comparable effect of TZDs regardless of diabetes duration or baseline cardiovascular risk. Moreover, the finding of an association between TZD monotherapy and adverse events suggests that this selection bias does not explain our results. We also minimized this concern by using other oral hypoglycemic agent combination therapies as the reference group, by subdividing TZD and other drug exposure groups, and by adjusting for prior diabetes treatments.
In summary, in this population-based study of older community-dwelling patients with diabetes, TZD treatment was associated with a significant increase in the risks of CHF, AMI, and death compared with other oral hypoglycemic agent treatments. These findings provide evidence from a real-world setting and support data from clinical trials that the harms of TZDs may outweigh their benefits, even in patients without obvious baseline cardiovascular disease. Further studies are needed to better quantify the risk-benefit trade-offs associated with TZD therapy and to explore whether the hazards associated with these agents are specific to rosiglitazone. In the interim, treatment decisions must remain individualized, with clinicians weighing the potential benefits and harms of TZD treatment, especially among high-risk elderly populations.
INTRODUCTION
Cardiovascular disease is an important cause of morbidity and mortality among persons with type 2 diabetes mellitus.1-3 The thiazolidinediones (TZDs), rosiglitazone and pioglitazone, are oral hypoglycemic agents that have been shown to improve glycemic control and may act to slow the progression of beta cell failure.4 While improved glycemic control has been linked to better clinical outcomes in diabetes5-7 and TZDs have been suggested as having potential cardiovascular benefits,8-11 recent concerns have arisen regarding adverse cardiac effects of these drugs.
Use of TZDs is associated with weight gain and edema,12 and evidence suggests that both rosiglitazone and pioglitazone increase the risk of congestive heart failure (CHF).8, 10, 13-19 A recent boxed warning for CHF was added for these agents recommending against the use of TZDs in persons with preexisting CHF.20 Two meta-analyses have also suggested that rosiglitazone may be associated with an increased risk of acute myocardial infarction (AMI) and death.18, 21 These findings prompted a hearing by a US Food and Drug Administration advisory panel regarding the safety of rosiglitazone; however, the panel voted against removing rosiglitazone from the market because of insufficient data.22
Most studies to date far have examined adverse cardiovascular outcomes associated with TZDs among clinical trial samples. However, the extent to which these adverse effects apply to real-world populations is less clear. In addition, older persons are traditionally underrepresented in clinical trials,23-24 and the majority of TZD trials were limited to persons younger than 65 years.8, 18-19,21 The necessity for evaluating TZD-associated outcomes in older patients is further reinforced by the fact that those aged 65 years or older have the highest prevalence of diabetes and represent more than 40% of the population with diabetes.25 Moreover, both the baseline risk of adverse cardiovascular events and the risk of medication-related harms are likely greater among older than younger populations. Accordingly, the objective of this study was to evaluate the risks of CHF, AMI, and all-cause mortality associated with TZD use compared with alternative oral hypoglycemic agents among patients aged 66 years or older with diabetes in Ontario.
METHODS
Overview
We conducted a population-based, retrospective cohort study that was analyzed using a nested case-control approach. Among a cohort of older patients with diabetes treated with oral hypoglycemic agents, we used Ontario health care databases to explore the association between 3 distinct outcomes (hospital visits for CHF, hospital visits for AMI, and deaths from any cause) and exposure to specific oral hypoglycemic agents. This project was approved by the institutional review board of Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Context
The Ontario Drug Benefit program covers medication costs for patients aged 65 years or older. During the time of this study, reimbursement for TZDs was restricted to patients whose glycemia was uncontrolled by or had a contraindication or intolerance to metformin and/or sulfonylureas. To receive coverage, clinicians submit a standardized request form, and an individual internal review process is undertaken. Approximately 10% to 13% of requests were rejected during our study period (personal communication, Mona Sabharwal, BScPHM, PharmD, Ontario Drug Benefit program, October 29, 2007).
Data Sources
We used health databases from Ontario, Canada's largest province, with a population of 12 259 568 at the mid point of the study period, including 1 457 408 who were aged 66 years or older. We identified prescription records using the Ontario Drug Benefit database, which contains records of all publicly funded medications dispensed to Ontarians aged 65 years or older.26 Hospital visits were identified using the National Ambulatory Care Reporting System database and the Canadian Institute for Health Information Discharge Abstract Database, which detail emergency department visits and hospital admissions, respectively. The Ontario Health Insurance Plan database was used to identify physician service claims. We obtained demographic information and deaths from the Registered Persons Database and diabetes status from the Ontario Diabetes Database.27 These databases were linked anonymously using encrypted health card numbers.28-30
Study Population and Cohort Definition
The source population consisted of all residents aged 66 years or older with diabetes as identified in the Ontario Diabetes Database and who were dispensed at least 1 oral hypoglycemic agent between April 1, 2002, and March 31, 2005. The cohort entry date was defined as the date of the first prescription for an oral hypoglycemic agent, including TZDs (rosiglitazone or pioglitazone), metformin, sulfonylureas, and other oral agents (ie, meglitinides or acarbose). Because patients receiving insulin tend to have either more advanced diabetes or type 1 diabetes and have also been shown to have a higher rate of CHF compared with TZD-treated patients,31 we excluded patients who received insulin in the year preceding cohort entry. However, patients who started receiving insulin during follow-up were retained in the study. For each outcome, we followed up eligible individuals until they experienced an event, death, a last health services contact in Ontario (for those who lost health contact for at least 6 months), or March 31, 2006, whichever came first.
Study Outcomes
The primary outcome measure was a first hospital visit for CHF, defined as either an emergency department visit for CHF or a hospital admission with a discharge diagnosis of CHF (International Classification of Diseases, 10th Revision [ICD-10] code I50). Secondary outcomes included a hospital visit for AMI and all-cause mortality. AMI was defined as either an emergency department visit for AMI or a hospitalization with a discharge diagnosis of AMI (ICD-10 codes I21, I24, and I25.4). The date of admission or emergency department visit for each outcome was taken as the index date. Deaths from any cause were identified using the Registered Persons Database and the hospital discharge abstract database, and the date of death was taken as the index date.
Selection of Cases and Controls
For each outcome (CHF, AMI, and all-cause mortality), cases were defined as all individuals from the cohort who had an event during their follow-up period. Potential controls for each case were persons who were still at risk for the event on the index date for that case, in that they were alive, remained in the province, and were event-free until the index date. Controls were assigned the same index date as their respective cases. For each case, up to 5 controls were randomly selected and matched on age (±1 year), sex, diabetes duration (<2 years, 2-5 years, or 5 years), and history of cardiovascular disease within 5 years of cohort entry. In the CHF and AMI analyses, controls were also matched on history of an event (within 1 year of cohort entry and within 1-5 years).
Exposure to Hypoglycemic Drugs
All hypoglycemic drugs (oral hypoglycemic agents and insulin) covered by the Ontario Drug Benefit program during the study period were identified and classified into 6 mutually exclusive groups: (1) TZD monotherapy (subdivided into rosiglitazone and pioglitazone); (2) other oral hypoglycemic agent monotherapy (metformin, sulfonylurea, acarbose, or a meglitinide); (3) TZD combination therapy (TZD and at least 1 other agent); (4) other oral hypoglycemic agent combination (2 or more non-TZD agents); (5) insulin monotherapy; and (6) insulin combination therapy (excluding TZDs).
Thiazolidinedione monotherapy was our primary exposure of interest, as this group was more likely to have received coverage because of a contraindication or intolerance to other oral hypoglycemic agents rather than inadequate glycemic control requiring the addition of a TZD. The association between TZDs and cardiovascular risk was thus less likely to be affected by diabetes severity or other concurrent oral hypoglycemic agents in monotherapy users.
Statistical Analysis
Because medication use changes over time, we analyzed drug exposure as a time-dependent variable. We used a nested case-control analysis of the cohort to evaluate the association between TZD exposure and each outcome because of the large cohort size and relatively long follow-up.32-33 This approach has been shown to result in significant gains in computational efficiency while providing unbiased estimates of the rate ratios (RRs) that would be obtained from a traditional time-to-event analysis of the full cohort, with little or no loss in precision.34-36
Primary Analysis
For each analysis, we examined drug exposures in the year preceding the index date. The duration of each prescription was determined from the mandatory "days supply" field of the Ontario Drug Benefit record (the number of days for which the drug was prescribed), and 14 days were added each prescription to accommodate nonadherence. Based on the most proximate prescription before the index date, patients were characterized as current users if their prescription duration encompassed the index date and past users if their last prescription ended in the 15 to 365 days preceding their index date. Current users included all persons who were taking the drug of interest at the time of the event, regardless of treatment duration. Persons with no hypoglycemic prescriptions in the year preceding the index date were classified as nonusers.
We compared the risk of CHF, AMI, and all-cause mortality associated with current use of TZDs (separately for monotherapy and in combination) with that of other oral hypoglycemic agent combination therapy. The latter was chosen as the reference category because patients treated with combination oral hypoglycemic agent therapy are likely to be most similar (in terms of diabetes severity and progression) to individuals treated with TZDs, given the prescribing restrictions applied to the TZDs. We estimated unadjusted and adjusted RRs for these associations using conditional logistic regression.37-38 All RRs were adjusted for exposure to other hypoglycemic drugs, past TZD use, and potential confounders such as sociodemographic factors, well-known risk factors for each outcome, concomitant use of cardiovascular medications, previous use of oral hypoglycemic agents, and 2 indices of health status (the Charlson index39 and the number of distinct drugs dispensed40). Individuals with missing data were given a separate "missing" classification for those variables for inclusion in adjusted models.
Additional Analyses
To investigate the potential risk-modifying effects of preexisting cardiovascular disease and diabetes duration (<5 vs 5 years), we included an interaction term for each TZD exposure group and history of cardiovascular disease in subsequent models. To assess the robustness of our conclusions, we explored the association between each outcome and use of antibiotic eye drops because these drugs are not plausibly associated with the study outcomes. All analyses were conducted using SAS version 9.1 (SAS Institute Inc, Cary, North Carolina) and used a 2-tailed P value of .05 as the threshold for statistical significance.
Estimate of Absolute Risks of TZD Use
To estimate the absolute risk associated with TZDs for CHF, AMI, and death, we first calculated the event rates over 4 years for all cohort patients who were alive and remained in Ontario until the end of the study period. We applied the RR estimates for TZD treatment from the nested case-control analysis to the baseline event rates in the cohort population to estimate the number needed to harm for AMI, CHF, and death. Although these RRs were calculated based on comparison with the non-TZD combination therapy users, we assumed that their event rates were at least as high as for the entire cohort.
RESULTS
The study population consisted of 159 026 individuals with oral hypoglycemic agent-treated diabetes with a mean age of 74.7 years and who were followed up for a median of 3.8 years. During this time, 7.9% of patients had a hospital visit for CHF (n = 12 491), 7.9% had a visit for AMI (n = 12 578), and 19% died (n = 30 265). The majority of CHF and AMI cases were recorded from hospitalization abstracts; 17.5% of CHF and 3.1% of AMI cases were identified from emergency department records. Follow-up was 100% complete for patients who remained in Ontario during the study period.
The baseline characteristics of the original cohort by oral hypoglycemic agent exposure in the year prior to cohort entry are shown in Table 1. A higher proportion of individuals prescribed TZD monotherapy had a history of renal disease and cardiovascular disease compared with those prescribed TZD combination therapy and other oral hypoglycemic agent combination therapy. Individuals prescribed rosiglitazone monotherapy had greater comorbidity compared with those prescribed pioglitazone monotherapy; however, a similar proportion of individuals prescribed both TZD drug therapies had a history of cardiovascular disease. All other baseline characteristics were similar between exposure groups.
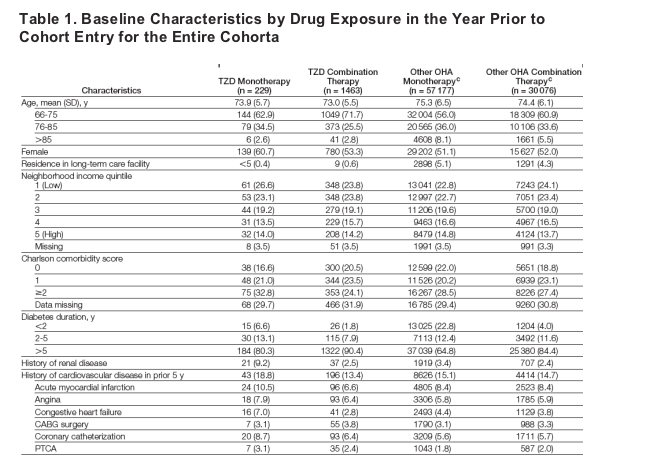
Baseline Data for Cases and Controls
The characteristics of cases of CHF, AMI, and deaths and their matched controls are shown in Table 2. Cases and controls were well matched with regard to age, sex, cardiovascular history, and diabetes duration, although the prevalence of noncardiac comorbidity was modestly greater among cases compared with controls. Collinearity between potential confounders was assessed, and all variance inflation factors were less than 3, indicating no evidence of collinearity between the covariates included in these models.
Association Between TZDs and CHF
Compared with oral hypoglycemic agent combination therapy users, current users of TZD monotherapy were at increased risk of CHF (78 cases; adjusted RR, 1.60; 95% confidence interval [CI], 1.21-2.10; P < .001), as were patients treated with TZD combination therapy (508 cases; adjusted RR, 1.31; 95% CI, 1.17-1.47; P < .001) (Table 3). This increased risk was identified only with rosiglitazone, regardless of whether it was used as monotherapy (62 cases; RR, 1.98; 95% CI, 1.44-2.72; P < .001) or in combination with other agents (364 cases; RR, 1.43; 95% CI, 1.25-1.63; P < .001). Although the point estimate for pioglitazone does not favor a comparable increased risk, we had limited power to explore the association between outcomes and pioglitazone use because of smaller numbers of patients receiving this agent. Past TZD use was also associated with a higher risk of CHF (Table 3), which may have been due to either a residual effect of TZDs or discontinuation in persons with early signs of CHF.
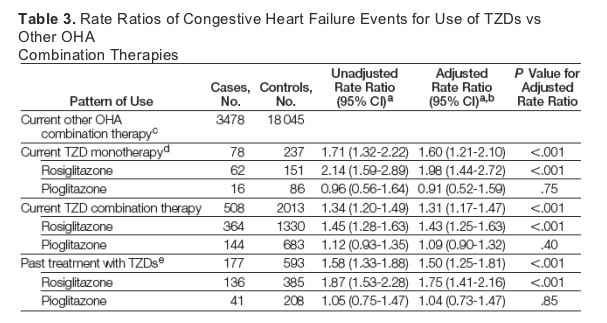
Stratified analyses by diabetes duration indicated no difference in CHF risk for those with less than 5 years of diabetes duration (RR, 1.75; 95% CI, 1.03-2.98) vs 5 years or longer (RR, 1.55; 95% CI, 1.13-2.12; P = .70 for interaction). We had insufficient power to explore the association of a history of CHF with the relationship between CHF and TZD therapy, perhaps because physicians generally avoided these drugs in such patients. However, even among patients with no history of CHF, a significant association with CHF risk remained for patients treated with TZD monotherapy (RR, 1.70; 95% CI, 1.27-2.26) and combination therapy (RR, 1.35; 95% CI, 1.20-1.53).
Association Between TZDs and AMI
Thiazolidinedione treatment was also associated with an increased risk of AMI (Table 4). Current users of TZD monotherapy were at increased risk of AMI vs users of other oral hypoglycemic agent combinations (65 cases; adjusted RR, 1.40; 95% CI, 1.05-1.86; P = .02). However, we did not observe an increased risk of TZD combination therapy (404 cases; RR, 0.96; 95% CI, 0.85-1.08; P = .49). As observed in the CHF analysis, the association between AMI and TZD monotherapy appeared to be limited to rosiglitazone (53 cases; RR, 1.76; 95% CI, 1.27-2.44; P < .001). Subgroup analyses indicated no difference in treatment effect based on diabetes duration (<5 years: RR, 2.00; 95% CI, 1.12-3.56; 5 years: RR, 1.26; 95% CI, 0.91-1.75; P = .17 for interaction) or history of AMI (no AMI: RR, 1.41; 95% CI, 1.04-1.92; AMI history: RR, 1.29; 95% CI, 0.57-2.90; P = .83 for interaction).
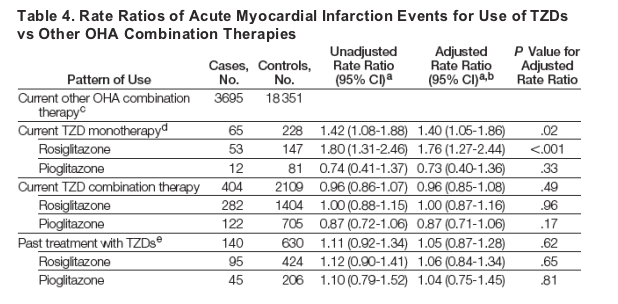
Association Between TZDs and Mortality
Both TZD monotherapy and combination therapy were associated with an increased risk of death compared with other oral hypoglycemic agent combination therapies (monotherapy: 102 cases; RR, 1.29; 95% CI, 1.02-1.62; P = .03; combination therapy: 497 cases; RR, 1.24; 95% CI, 1.11-1.39; P < .001). Again, this association was significant only with rosiglitazone therapy (Table 5). Past TZD treatment was also associated with higher mortality (Table 5), possibly because of a residual effect, early discontinuation of these agents in terminally ill patients, or both. There was no significant interaction between TZD use and diabetes duration or cardiovascular history in the mortality analysis.
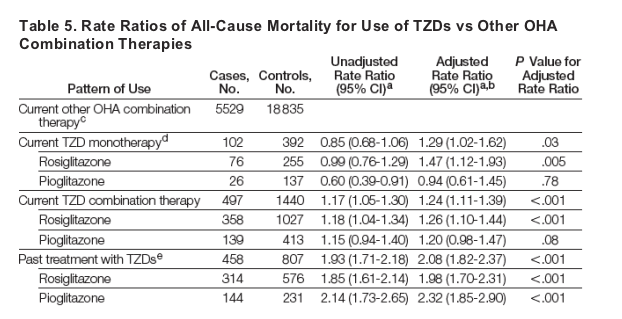
Other Drug Exposure
Compared with non-TZD oral hypoglycemic agent combination therapy, oral hypoglycemic agent monotherapy (eg, metformin or sulfonylurea) was associated with no increase in CHF (RR, 0.96; 95% CI, 0.91-1.02) or AMI (RR, 1.00; 95% CI, 0.95-1.05) but a decrease in deaths (RR, 0.54; 95% CI, 0.52-0.56; P < .001). Like TZD users, persons who started insulin during the follow-up period had a higher risk of CHF, AMI, and death (insulin monotherapy: RR for CHF, 1.80; 95% CI, 1.59-2.05; P < .001; RR for AMI, 1.52; 95% CI, 1.32-1.74; P < .001; RR for death, 1.92; 95% CI, 1.78-2.07; P < .001). However, insulin users also had higher comorbidity (54.4% with a Charlson score of 2) and history of cardiovascular disease (43.8%), CHF (14.9%), and renal disease (11.6%) compared with TZD users. As expected, we did not observe an increased risk of events among users of antibiotic eye drops compared with patients not receiving these drugs.
Absolute Risk of CHF, AMI, and Death
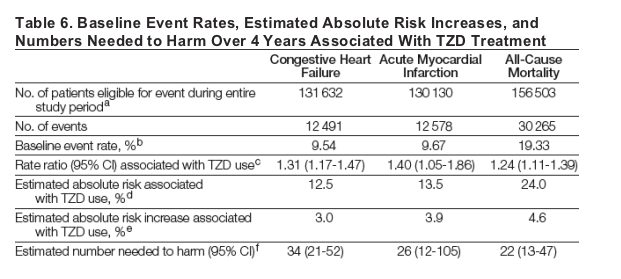
After excluding those who died or had a lost health contact before the end of the study period for each outcome, the baseline event rates over the 4-year follow-up were 9.54% for CHF, 9.67% for AMI, and 19.33% for death. Based on these rates and the RRs associated with TZD use, the estimated numbers needed to harm over 4 years were 34 (95% CI, 21-52) for CHF, 26 (95% CI, 12-105) for AMI, and 22 (95% CI, 13-47) for death (Table 6).
|
|
| |
| |
|
|
|