 |
 |
 |
| |
Long-term Entecavir Therapy Results in Reversal of Fibrosis/Cirrhosis and Continued Histologic Improvement in Patients with HBeAg(+) and (-) Chronic Hepatitis B: Results from Studies ETV-022, -027 and -901
|
| |
| |
Reported by Jules Levin
AASLD Nov 2 2008, San Francisco, CA
Yun-Fan Liaw1, Ting-Tsung Chang2, Shun-Sheng Wu3, Eugene R. Schiff4, Kwang-Hyub Han5, Ching-Lung Lai6, Rifaat Safadi7, Samuel S. Lee8, Waldemar Halota9,
Zachary D. Goodman10, Hui Zhang11, Robert Hindes11, Uchenna Iloeje11, Suzanne Beebe11, Bruce Kreter12
1Chang Gung Memorial Hospital, Chang Gung University College of Medicine,
Taipei, Taiwan; 2National Cheng Kung University Medical College, Tainan, Taiwan; 3Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan; 4University of Miami Hospital & Clinics, Miami, FL, USA; 5Department of Internal Medicine, Yonsei University College
v
of Medicine, Seoul, South Korea; 6Department of Medicine, Queen Mary's Hospital, University of Hong Kong, Hong Kong, China; 7Division of Medicine, Hadassah Medical Center, Jerusalem, Israel; 8Liver Unit, University of Calgary, Calgary, AB, Canada; 9Klinika Chorob Zakaznych AM, Bydgoszcz, Poland; 10Armed Forces Institute of Pathology, Washington, WA, USA;
11Research & Development, Bristol-Myers Squibb Company, Wallingford, CT, USA; 12Research & Development, Bristol-Myers Squibb Company, Princeton, NJ, USA
SUMMARY OF RESULTS
Ninety-six percent of patients in the Long-term Histology Cohort who received continuous treatment with ETV achieved histologic improvement
All patients with advanced fibrosis/cirrhosis at baseline (Ishak fibrosis score ≥4) demonstrated an improvement in fibrosis
At the time of long-term biopsy:
-- all patients had HBV DNA <300 copies/mL
-- eighty-six percent of patients had ALT <1 x ULN
Week 48 results for the Efficacy Evaluable Cohort were comparable to the respective Phase 3 parent studies (ETV-022 and ETV-027)
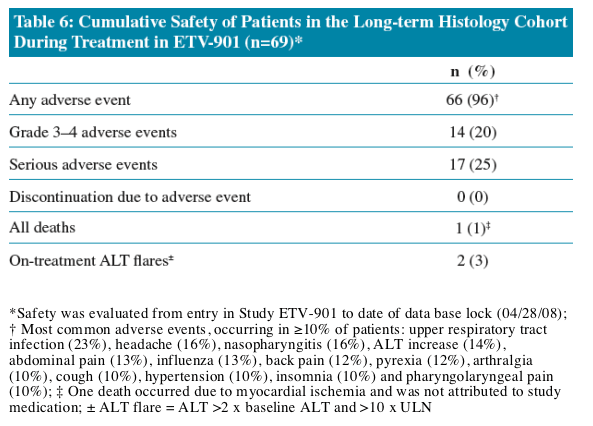
Safety profile was consistent with previously reported experience
CONCLUSION
The results from this cohort demonstrate that long-term ETV treatment result in durable suppression of viral replication and regression of fibrosis/cirrhosis
INTRODUCTION
Elevated baseline HBV DNA has been demonstrated to be a significant factor in the development of cirrhosis and hepatocellular carcinoma1-3
HBV DNA suppression with antiviral therapy can significantly improve liver histology in HBeAg(+) patients4
Entecavir (ETV) 0.5 mg daily demonstrated superior histologic, virologic and biochemical activity compared to lamivudine (LVD) 100 mg daily in nucleoside-naïve HBeAg(+) and HBeAg(-) patients with chronic hepatitis B (studies ETV-022 and ETV-027)5,6
We present long-term histologic results for a subset of patients treated with ETV for a median of 280 weeks
METHODS
Study population
· The Long-term Histology Cohort (n=69) is a subset of the ETV-901
study population
- It consists of nucleoside-naïve HBeAg(+) and HBeAg(-) patients treated with ETV in studies ETV-022 or ETV-027 who:
· had a liver biopsy in study ETV-901 and
· received a minimum of 3 years of cumulative ETV therapy from Phase 3 baseline to the time of their last observed biopsy in study ETV-901
· Patients received ETV 0.5 mg once daily in studies ETV-022 and -027
· All patients received ETV 1.0 mg in study ETV-901
- Initially, due to ongoing blinding of Phase 2-3 studies, patients enrolling into study ETV-901 may have received a brief period of combination ETV 1.0 mg and LVD 100 mg daily
Efficacy Evaluable Cohort
· The Efficacy Evaluable Cohort (n=57) consists of patients who had:
- an adequate Phase 3 baseline biopsy
- a baseline Knodell necroinflammatory score of ≥2
- an adequate long-term biopsy sample in study ETV-901
Analysis endpoints: liver histology
· Co-primary endpoints
- Histologic improvement (≥2-point decrease in Knodell necroinflammatory
score and no worsening of Knodell fibrosis score)
compared to baseline
- Improvement in Ishak fibrosis score (≥1-point decrease) compared with baseline
· Other histologic endpoints
- Change from baseline in Knodell necroinflammatory score
- Change from baseline in Ishak fibrosis score
- Proportion of patients with baseline advanced fibrosis/cirrhosis (Ishak score ≥4) who demonstrated Ishak score improvement
- Proportion of subjects with baseline histologic activity index (HAI) score of ≥4 who achieved a Knodell HAI score ≦3
Analysis endpoints: virologic, biochemical, serologic and safety
· All efficacy analyses were conducted on samples that matched the time of long-term biopsy (±12 weeks) and compared with Phase 3 baseline
· Proportions of patients with HBV DNA <300 copies/mL by PCR, alanine
aminotransferase (ALT) ≦1 x ULN, HBeAg loss, HBe seroconversion and HBsAg loss were assessed among patients with available samples (Non-completer=Missing)
· Safety was evaluated from entry in study ETV-901 to date of database lock (28 April 2008)
RESULTS
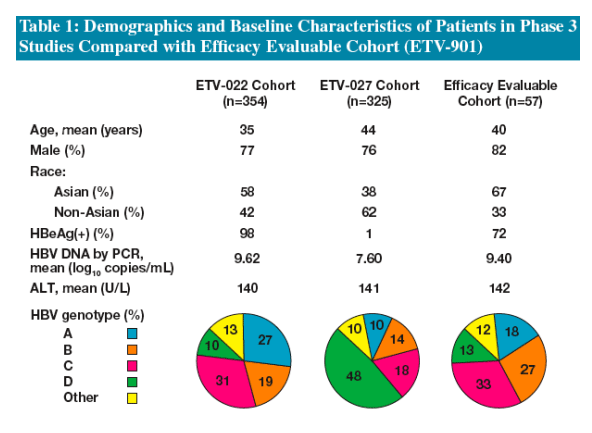
Demographics and baseline characteristics of the Efficacy Evaluable Cohort were generally comparable to those of all treated patients in studies ETV-022 and -027, with the exception of the higher proportion of Asians in the Efficacy Evaluable Cohort
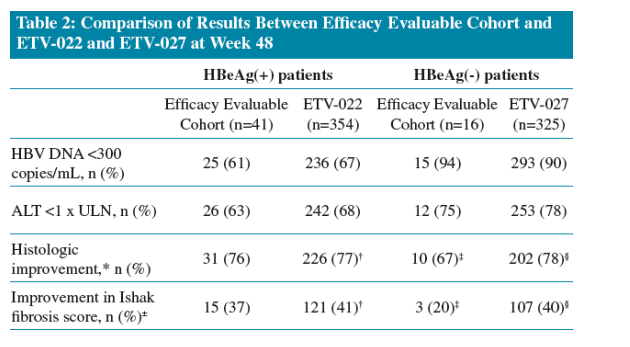
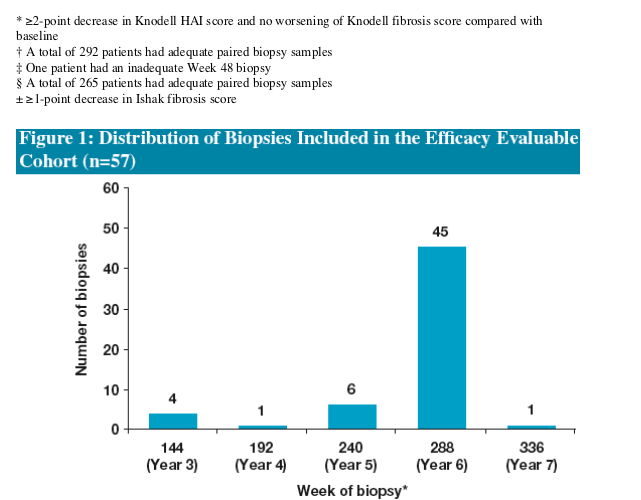
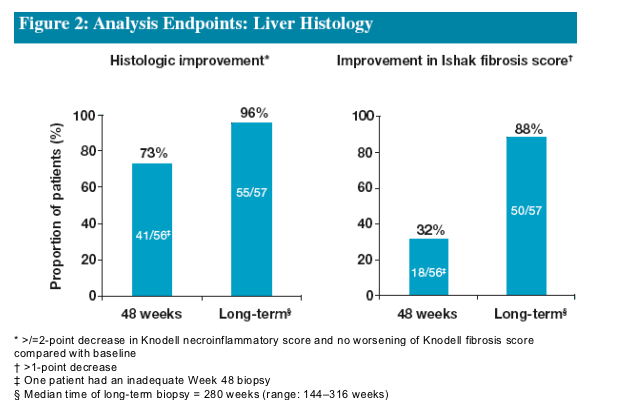
Following 48 Weeks of ETV treatment the majority (73%) of patients achieved histologic improvement
- The proportion of patients who achieved histologic improvement increased to 96% following long-term treatment
Improvement in Ishak Fibrosis score was observed in 32% of patients following 48 Weeks of ETV treatment
- This increased to 88% following long-term treatment
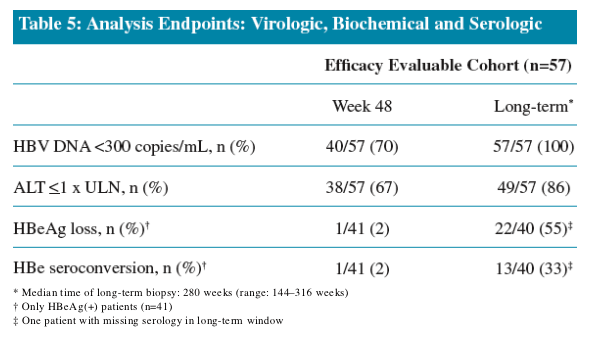
All patients achieved undetectable HBV DNA (<300 copies/mL) at the time of long-term biopsy
Increasing proportions of patients achieved ALT normalization (ALT <1 x ULN) with long-term ETV treatment
Patient management criteria in ETV-022 mandated that patients with
HBeAg loss and HBV DNA <0.7 MEq/mL discontinue study therapy
and be followed off-treatment. Most patients who achieved HBeAg
loss in ETV-022 were therefore not included in the Long-termHistology Cohort
No patients in the Evaluable Efficacy Cohort lost HBsAg
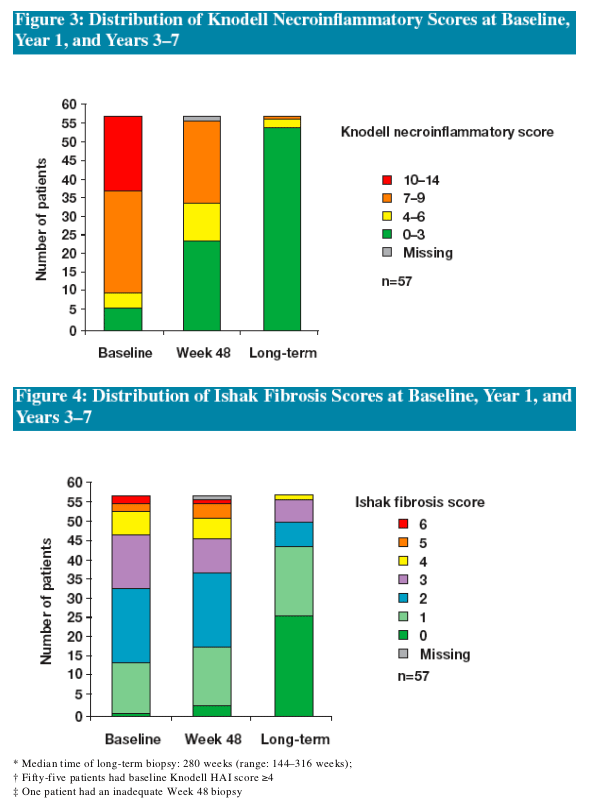
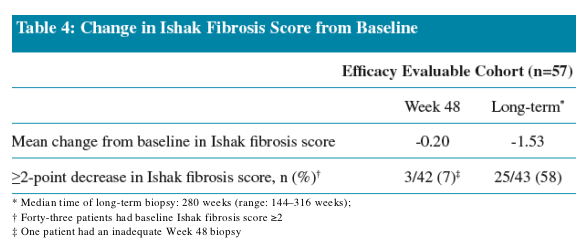
Reversal of advanced fibrosis/cirrhosis
Ten patients had baseline advanced fibrosis/cirrhosis (Ishak fibrosis score = 4, 5 or 6)
- All demonstrated an improvement in Ishak fibrosis score (≥1-point improvement)
- Four patients had a liver biopsy with cirrhosis at baseline; all demonstrated an improvement of Ishak fibrosis score
The median change in Ishak fibrosis score was a 3-point decrease (range: -1 to -4)
REFERENCES
[1] Chen CJ, Yang HI, Su J, et al. JAMA 2006;295:65-73. [2] Iloeje UH, Yang HI, Su J, et al. Gastroenterology 2006;130:678-686 [3] Chen G, Lin W, Shen F, et al. Am J Gastroenterol 2006;101:1797-1803 [4] Mommeja-Marin H, Mondou E, Blum MR, et al. Hepatology 2003;37:1309-1319 [5] Chang TT, Gish RG, de Man R, et al. N Engl J Med 2006;354:1001-1010 [6] Lai CL, Shouval D, Lok AS et al.. N Engl J Med 2006;354:1011-1020
|
| |
|
 |
 |
|
|