 |
 |
 |
| |
Adherence with Lopinavir/ritonavir (LPV/r) Tablet and Soft-Gel Capsule (SGC)-Based Antiretroviral Regimens and Predictors of Early Treatment Compliance
|
| |
| |
Reported by Jules Levin
R Rode PhD, T Marsh, C Naylor, D Cohen MD, T Podsadecki MD
Abbott Laboratories, Abbott Park, IL, USA , HIV 9, 9-13 November 2008 ♦ Glasgow, UK
Author Conclusions
During the first 12 weeks of randomized therapy, LPV/r QD dosing resulted in higher levels of adherence than BID dosing.
In the setting of this clinical trial, adherence to LPV/r tablets and SGC was similar. Of note, because MEMS caps were used, subjects were required to
dose LPV/r from the original container which could have impacted potential differences in adherence related to the convenience of the LPV/r tablet.
Sex, age and race were predictors of early adherence.
Differences in early adherence at 12 weeks do not appear to predict clinical outcomes at 48 weeks.
The significant between-strata difference observed in CD4+ T-cell increases when comparing whites to non-whites was consistent over time and may, in part, reflect the baseline CD4+ T-cell count difference between whites and non-whites observed in this study.
BACKGROUND
Simplified antiretroviral (ARV) regimens may promote adherence and improve outcomes in HIV-1 infected patients.
Clinical studies have previously demonstrated greater adherence with LPV/r soft gel capsules (SGC) when dosed once-daily (QD) compared to twice-daily (BID).1
Previous studies have shown patient preference for the LPV/r tablet over the SGC primarily related to convenience (lack of refrigeration, reduction in pill count and dosing without regard to meals).2,3
The objectives of the current analysis are to compare adherence when LPV/r tablets and LPV/r SGC are dosed QD and BID and to assess predictors of early adherence.
METHODS
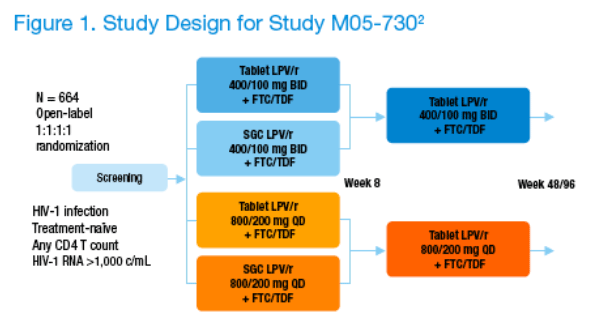
M05-730 is an ongoing Phase 3, open-label, randomized, multicenter, multi-country study designed to evaluate the safety, tolerability, pharmacokinetics, and antiviral activity of LPV/r tablets dosed QD or BID through 48 and 96 weeks in combination with once daily tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) in ARV-naïve HIV-1 infected subjects.
-- 664 ARV-naïve HIV-1-infected subjects were randomized 1:1:1:1 to LPV/r QD SGC, BID SGC, QD tablet, or BID tablet for 8 weeks. At Week 8, subjects taking LPV/r SGC were switched to the LPV/r tablet formulation while maintaining their QD or BID dosing schedule (Figure 1).
MEMS Monitoring
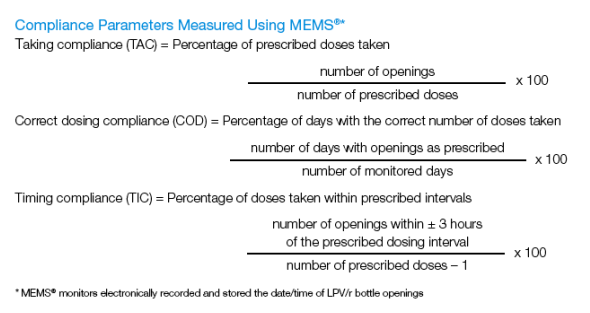
Electronic monitoring (Medication Event Monitoring System [MEMS]; AARDEX) allowed for computation of 3 LPV/r compliance measures through Week 12: taking (TAC; prescribed doses taken), correct dosing (COD; days with correct number of doses taken) and timing (TIC; doses taken within ± 3 hours of prescribed interval) compliance.
Statistical Methods
Demographic and baseline characteristics were evaluated using a one-way analysis of variance (ANOVA) for continuous measurements and Fisher's exact test for discrete measurements.
Longitudinal mixed-effects models were used to examine the potential association between treatment compliance (through Week 12) and demographic/baseline characteristics. Given that compliance was measured at equal intervals during the first 12 weeks (i.e., Baseline - Week 4, Week 4 - Week 8, and Week 8 to Week 12), a first-order autoregressive [AR(1)] variance-covariance structure was used.
Percentages of subjects with plasma HIV-1 RNA levels below 50 copies/mL were evaluated using Fisher's exact test. For the purpose of these analyses, an "Intent-to-Treat (ITT): Non-completer = Failure" assessment was used.
CD4+ T-cell count changes from baseline were evaluated over time using a mixed-effects model with baseline CD4+ T-cell count as a covariate and assuming an unstructured variance-covariance matrix.
RESULTS
Baseline Demographics
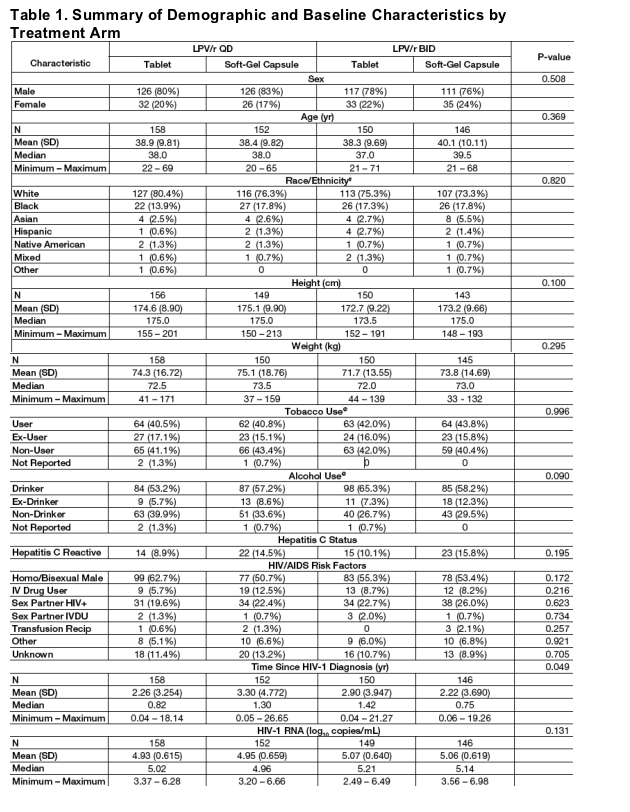
Of the 664 subjects enrolled, 606 (91%) have MEMS compliance data available and were included in this analysis.
Demographic and baseline characteristics were similar between treatment arms (Table 1).
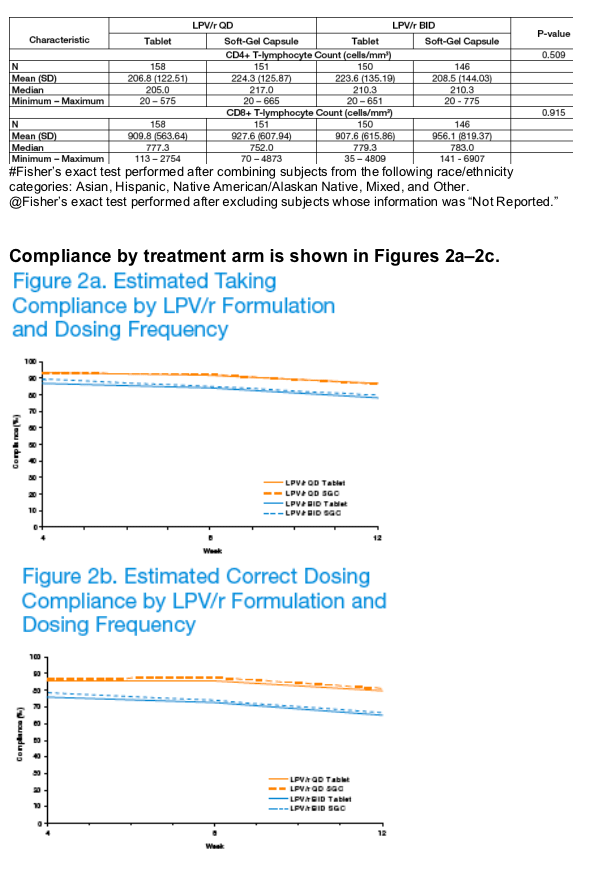
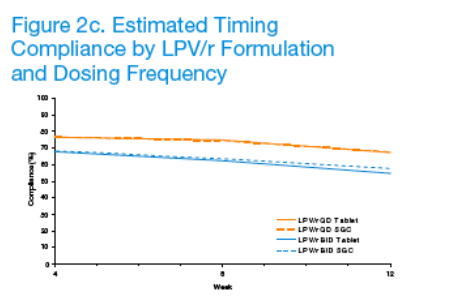
Adherence to LPV/r dosed QD was statistically significantly greater than BID (p <0.001).
Adherence declined significantly over time (p <0.001), but between-arm differences remained consistent over 12 weeks (p ≥0.353).
Adherence was similar for subjects taking LPV/r tablets compared to SGC.
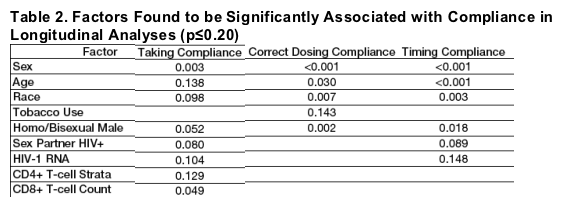
Sex, age, race, tobacco use, HIV/AIDS risk factors, HIV-1 RNA, and CD4+ and CD8+ T-cell count were significantly associated with early compliance (p≦0.20).
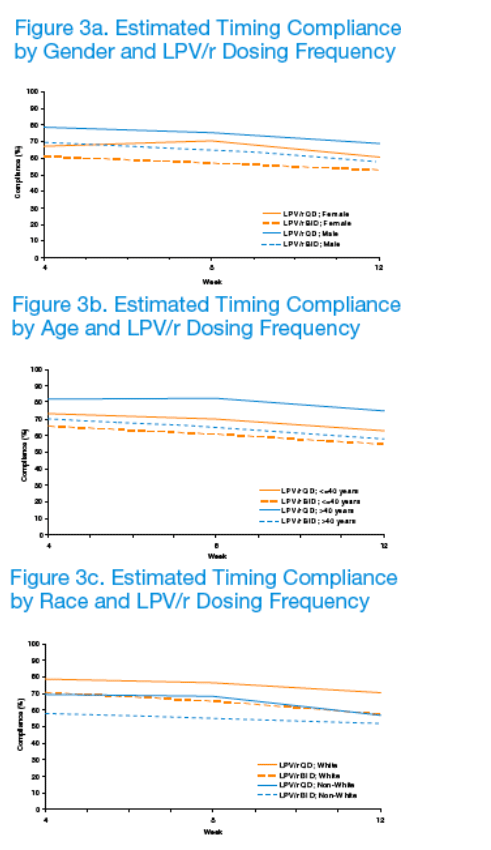
The strongest associations were for TIC and sex (M>F), age (older>younger) and race (white>non-white) (p≦0.003) (Table 2; Figures 3a-3c).
Week 48 clinical outcomes did not differ for subjects receiving LPV/r QD or BID. Given the statistically significant association between treatment compliance and sex, age, and race, subgroup analyses were conducted to evaluate potential differences in virologic response to LPV/r-based antiretroviral therapy (Figures 4a-4c).
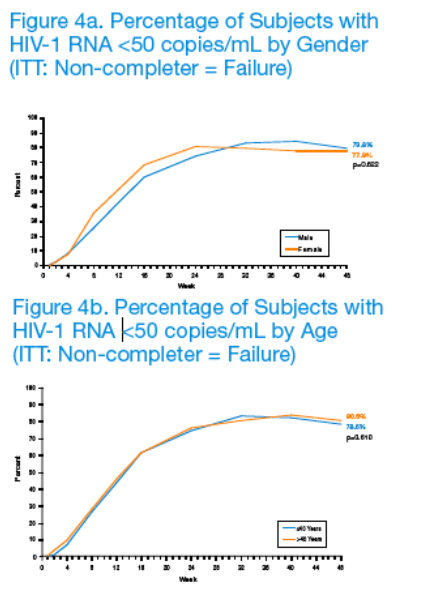
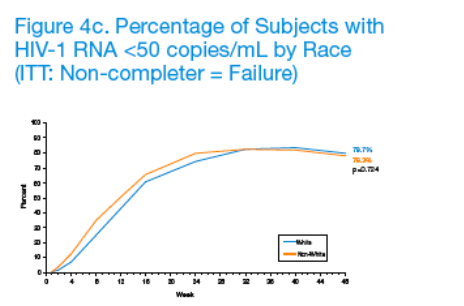
Based on results from these analyses, the percentages of subjects with plasma HIV-1 RNA <50 copies/mL at Week 48 did not appear to differ by sex (p=0.622), age (p=0.610) or race (p=0.724).
CD4+ T-cell counts significantly increased over time (p<0.001), with between-strata differences remaining consistent over 12 weeks (p≥0.262). Given the statistically significant association between treatment compliance and sex, age, and race, subgroup analyses were conducted to evaluate potential differences in immunologic response to LPV/r-based antiretroviral therapy (Figures 5a-5c).
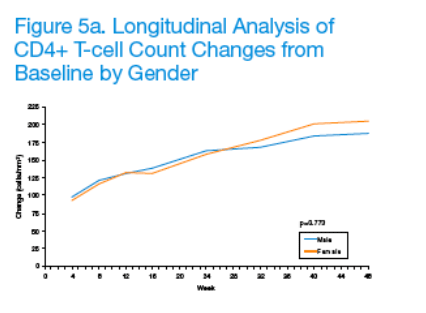
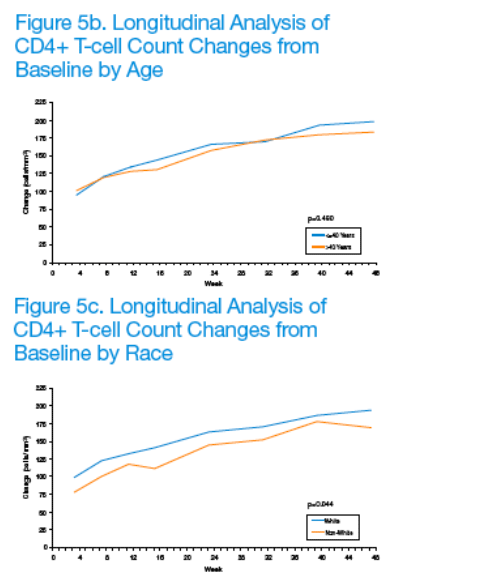
No significant between-strata differences were detected with respect to gender (male vs. female; p=0.773) and age (≦40 years vs. >40 years; p=0.480). However, a significant between-strata difference was detected with respect to race (white vs. non-white; p=0.044). In particular, the overall difference (mean ± SEM) between the two strata was 19.6 ± 9.67 cells/mm3, with whites tending to have higher mean CD4+ T-cell count changes than non-whites. Of note, however, whites also had higher baseline mean CD4+ T-cell counts than non-whites (222.5 ± 6.11 vs. 193.9 ± 10.99 cells/mm3; p=0.023).
References
1. Molina JM, Podsadecki TJ, Johnson MA, et al. A Lopinavir/Ritonavir-Based Once-Daily Regimen Results in Better Compliance and Is Non-inferior to a Twice-Daily Regimen Through 96 Weeks. AIDS Research and Human Retroviruses. 2007; 23:1505-1514.
2. Gathe J, Da Silva BA, Cohen D, et. al. A Once Daily Lopinavir/ritonavir Based Regimen Is Non-inferior to Twice Daily Dosing and Results in Similar Safety and Tolerability in Antiretroviral-Naïve Subjects through 48 Weeks. In Press. 2008.
3. Schrader S, Chuck S, Rahn L, et. al. Significant improvements in self-reported gastrointestinal tolerability, quality of life, patient satisfaction, and adherence with lopinavir/ritonavir after switching from BID soft-gel capsule to BID tablets. 8th IWADRLH, USA, 2006. Poster #81.
|
| |
|
 |
 |
|
|