 |
 |
 |
| |
Bone Mineral Density (BMD) 96 Weeks after Antiretroviral Therapy (ART) Initiation:
A Randomized Trial Comparing Efavirenz (EFV)-Based Therapy to a Lopinavir/ritonavir (LPV/r)-Containing Regimen with Simplification to LPV/r Monotherapy
|
| |
| |
Reported by Jules Levin
15th CROI, Feb 3-6, 2008, Boston
Brown TT1, McComsey GA2, King M3, Qaqish RB3, Bernstein B3, da Silva BA3
1Johns Hopkins University, Baltimore, Maryland, USA; 2Case Western Reserve University, Cleveland, Ohio, USA;
3Abbott, Abbott Park, Illinois, USA
15th Conference on Retroviruses and Opportunistic Infections (CROI) · February 3-6, 2008 · Boston, Massachusetts
AUTHOR DISCUSSION
· Prior to antiretroviral therapy initiation, lower baseline total BMD in HIV-infected persons was associated with lower weight and non-black race.
· The association between higher baseline BMD and higher levels of HIV-1 RNA was unexpected and may be attributable to unmeasured confounding factors.
· Higher baseline markers of inflammation were correlated with higher baseline HIV-1 viral load levels, but were not correlated with lower baseline BMD.
· Over 96 weeks of therapy, total BMD decreased by about 2.4% in both treatment groups, indicating that bone loss occurs upon initiation of antiretroviral therapy, regardless of whether PIs or NNRTIs are used.
· In vitro evidence has suggested that AZT increases osteoclastogenesis and promotes bone loss.2 However, in our study, discontinuation of AZT (with continuation of LPV/r monotherapy), did not attenuate the rate of BMD decline.
· Factors associated with bone loss of potential clinical significance (>5%) include low baseline CD4 count and non-black race. While higher fasting
glucose was associated with BMD loss, other parameters of glucose metabolism did not show a similar relationship to the change in BMD.
AUTHOR CONCLUSION
Similar decreases in total BMD over 96 weeks occurred in ART-naive subjects receiving either EFV or LPV/r-based regimens (~2.4%), which was not altered by simplification to LPV/r monotherapy. These data suggest that the loss of BMD with ART initiation occurs independently of the ART regimen used.
· Non-black subjects and those with lower nadir CD4 cell count may be at an increased risk of more pronounced BMD loss. (see table below showing relationship where BMD declines incrementally as CD4 count declines incrementally.)
ABSTRACT
Background: Reductions in bone mineral density (BMD) have been described in HIV-infected patients initiating ART. It is unclear whether this is due to an effect of ART or changes in immunologic function and/or viral activity. We compared changes in BMD from baseline (BL) to 96 weeks (wks) in subjects randomized to either a LPV/r simplification strategy or EFV+ ZDV/3TC. We also sought to identify factors associated with a >5% reduction in BMD.
Methods: 155 ART-naive HIV-1+ subjects were randomized (1:2) to receive EFV + ZDV/3TC (n=51) or LPV/r + ZDV/3TC induction (n=104) for 24-48 wks followed by LPV/r monotherapy (simplification). Subjects were followed for up to 96 wks with DEXA scans every 24 wks. Associations between BL total BMD and other BL factors were assessed by linear regression. Associations with a 5% decrease in total BMD through 96 wks were assessed by logistic regression. Factors tested included demographics, weight, HIV-1 RNA, CD4 cell count, smoking/alcohol history, body composition variables, HOMA-IR, TNF-alpha soluble receptors 1 and 2 (sTNFR).
Results:
74 LPV/r- and 32 EFV-treated subjects had DEXA scans available through 96 wks.
BL characteristics including mean ± SD total BMD were similar between groups: 1.18±0.10 grams/cm2 (LPV/r) and 1.19±0.12 grams/cm2 (EFV).
In a multivariable analysis, higher BL BMD was independently associated with higher weight, black race, and higher BL HIV-1 RNA (p<0.003 for each), but was not associated with age, smoking status, use of alcohol, CD4 cell count or sTNFR.
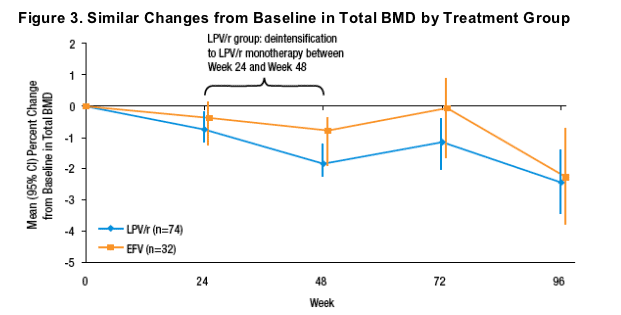
After 96 wks, mean percent change from baseline in total BMD was -2.5% (LPV/r) and -2.3% (EFV) (p<0.01 for within-group changes in either arm; p=0.86 for difference between groups).
No alteration in the rate of BMD change was observed upon simplification to LPV/r monotherapy.
Subjects with lower BL CD4 cell count, non-black race, and higher BL fasting glucose demonstrated a higher risk for >5% decrease in total BMD.
Change in total BMD through 96 wks was not correlated with BL BMD, other
parameters of glucose metabolism or changes in body composition.
Conclusions:
Similar decreases in total BMD over 96 wks occurred in ART-naive subjects receiving either EFV or LPV/r-based regimens, which was not altered by simplification to LPV/r monotherapy. These data suggest that the loss of BMD with ART initiation occurs independently of the ART regimen used. Non-black subjects and those with lower nadir CD4 cell count may be at increased risk of more pronounced BMD loss.
nbsp;
BACKGROUND
In cross-sectional studies, the prevalence of osteoporosis in HIV-infected individuals was found to be more than 3-fold higher than non-HIV-infected individuals.1
In addition, antiretroviral-treated subjects appeared to have an increased risk of decreased bone mineral density (BMD) and osteoporosis compared to antiretroviral-naive subjects.
While these data may suggest that both HIV and antiretroviral treatment impact BMD, longitudinal studies are required to understand the relative contributions of HIV infection and its treatment to decreased BMD observed in HIV-infected patients.
Longitudinal studies of HIV-infected subjects initiating antiretroviral therapy may be particularly informative in determining whether antiretroviral therapy is associated with reductions in BMD and whether some regimens have a greater impact than others.
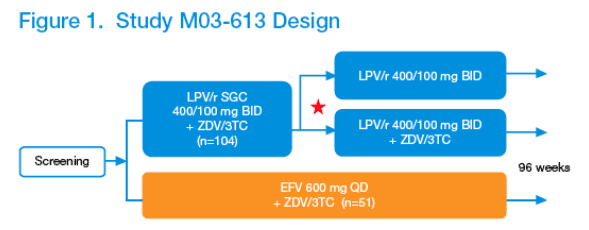
Study 613 is a 96-week prospective study in previously antiretroviral-naive subjects who were randomized to initiate either a protease inhibitor (PI) LPV/r-based regimen or an NNRTI efavirenz-based regimen. Those randomized to LPV/r and who met protocol specified criteria were later simplified to LPV/r monotherapy. Thus, this study allows us to evaluate BMD prospectively in a PI versus an NNRTI-based regimen and evaluate whether removal of the NRTI backbone impacts BMD. In addition, we also sought to identify factors associated with a potentially clinically significant BMD reduction [i.e. >5% (~0.6 SD)].
METHODS
155 antiretroviral-naive HIV-positive subjects were randomized to LPV/r+ZDV/3TC induction (n=104) for 24-48 weeks followed by LPV/r monotherapy if 3 consecutive HIV-RNA values <50 copies/mL were achieved, or to EFV+ZDV/3TC (n=51) for the entire study period. Overall study duration was 96 weeks (Figure 1).
Inclusion criteria
· Antiretroviral-naive
· HIV-1 RNA >1000 copies/mL
· Any CD4 count
· No evidence of resistance to study drugs
Kaletra Monotherapy Deintensification starting at Week 24 if 3 consecutive monthly HIV-1 RNA levels <50 copies/mL
92/104 subjects randomized to LPV/r + ZDV/3TC achieved 3 consecutive HIV-1 RNA levels <50 copies/mL, and then simplified to LPV/r monotherapy, and were followed for a median of 68 weeks on monotherapy. The majority of subjects simplified to LPV/r monotherapy at Week 24.
Subjects were followed for up to 96 weeks with DEXA scans every 24 weeks.
Associations between baseline total BMD and other baseline factors were assessed by linear regression.
Associations with a 5% decrease in total BMD through 96 weeks were assessed by logistic regression. Factors tested included demographics, weight, standard chemistry lab parameters (e.g., alkaline phosphatase), HIV-1 RNA, CD4 cell count, smoking/alcohol history (user/ex-user vs. non-user and drinker/ex-drinker vs. non-drinker), body composition variables (e.g., trunk fat, limb fat), HOMA-IR, TNF-alpha soluble receptors 1 and 2 (sTNFR I and II), and glucose and insulin area under the curve (AUC) during a 2-hour oral glucose tolerance test (OGTT).
RESULTS
Baseline Characteristics
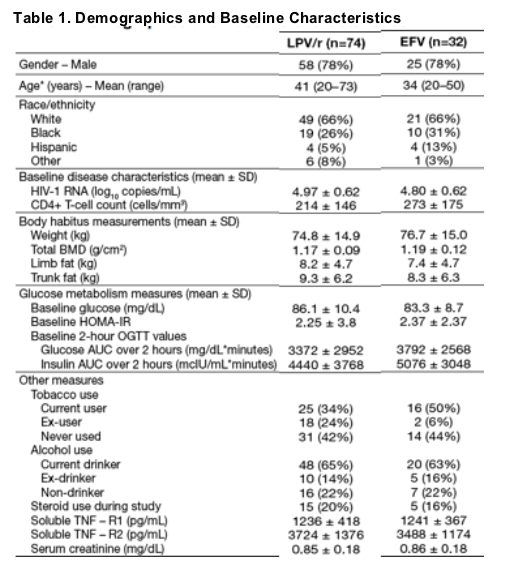
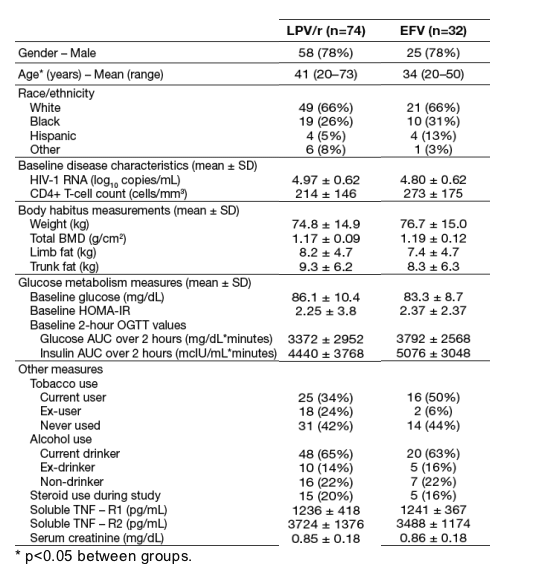
· Demographics and baseline characteristics were generally comparable within treatment groups (Table 1).
· 74 LPV/r- and 32 EFV-treated subjects had DEXA scans available through 96 weeks.
· Mean total BMD at baseline was 1.17±0.10 g/cm2 (LPV/r) and 1.19±0.12 g/cm2 (EFV) (p=ns).
Associations with Baseline BMD
· In a multivariable analysis, lower baseline total BMD was statistically significantly associated with lower weight, non-black race and lower baseline HIV-1 RNA levels (Table 2).
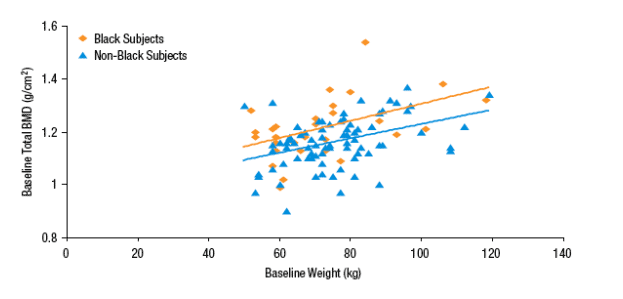
· The relationship between weight and total BMD is shown by race in Figure 2.
Table 2. Relationship Between Baseline BMD and Other Baseline Characteristics
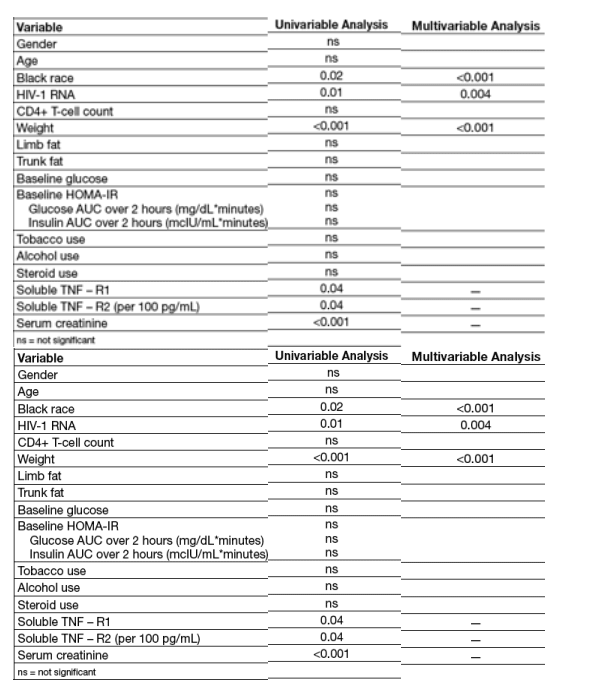
· Although higher baseline HIV-1 viral load was associated with black race, the positive association between baseline HIV-1 viral load and baseline BMD remained statistically significant after adjustment for race.
· Higher baseline HIV-1 viral load correlated with higher soluble TNF R1 and R2.
Figure 2. Baseline BMD is Positively Correlated with Black Race and Baseline Weight
Changes from Baseline in BMD
· After 96 weeks, similar mean decreases from baseline in BMD were observed by both treatment groups: -2.5% in the LPV/r group and -2.3% in the EFV group (p=0.86 for the difference between groups, Figure 3). Within treatment group, changes from baseline in BMD were statistically significant (p<0.01 for each group).
· No alteration in the rate of BMD change was observed when subjects in the LPV/r group simplified to LPV/r monotherapy (Figure 3).
· 16 subjects (15%) demonstrated a >5% decrease in BMD over 96 weeks.
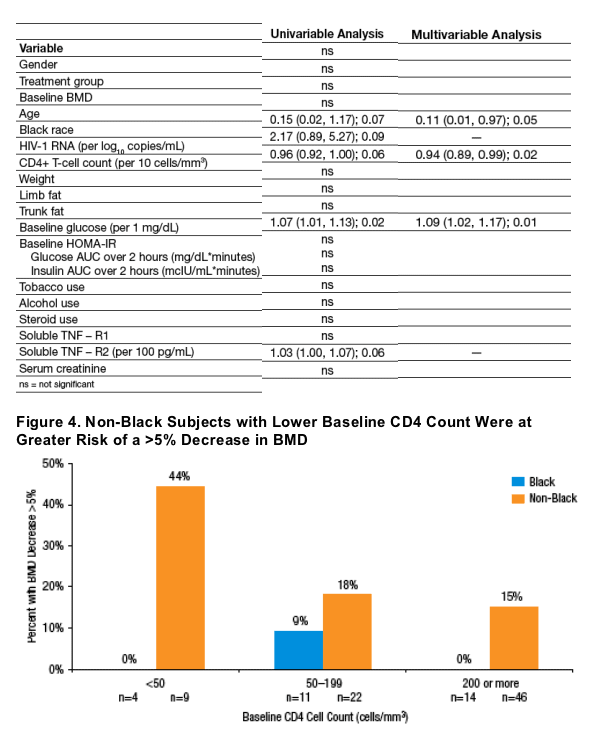
· Subjects with lower baseline CD4 cell count, higher baseline fasting glucose and of non-black race demonstrated a higher risk for >5% decrease in BMD (Table 3, Figure 3). Changes in BMD were not correlated to baseline BMD, other parameters of glucose metabolism or changes in body composition.
· Figure 4 illustrates that within each CD4 cell count strata assessed, a higher proportion of non-black subjects had a >5% decrease in BMD.
Table 3. Relationship Between Baseline Characteristics and the Risk of a 5% or Larger Decrease in BMD Over 96 Weeks (All Subjects)
REFERENCES
1. Brown, T. T. and Qaqish, R. B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: A meta-analytic review. AIDS 2006;20:2165-2174.
2. Pan G, Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM. AZT enhances osteoclastogenesis and bone loss. AIDS Res Hum Retroviruses 2004:608-20.
AIDS Research and Human Retroviruses
AZT Enhances Osteoclastogenesis and Bone Loss
George Pan, Xiaojun Wu, Margaret A. McKenna, Xu Feng, Tim R. Nagy, Jay M. McDonald. AIDS Research and Human Retroviruses. June 1, 2004, 20(6): 608-620. doi:10.1089/0889222041217482.
ABSTRACT. A variety of metabolic complications have been reported to be associated with highly active antiretroviral therapy (HAART), including osteopenia and osteoporosis. In this study, we determine the effects of zidovudine (AZT), a nucleoside reverse transcriptase inhibitor, on osteoclastogenesis in a cultured mouse macrophage preosteoclast cell line (RAW264.7), in mouse primary bone marrow macrophage-monocyte precursors, and on bone mineral density in mice. The results indicate that AZT induces an increase in osteoclastogenesis in the mouse preosteoclast cell line and in mouse bone marrow osteoclast precursors in the presence of RANKL. This increased osteoclastogenesis is dependent upon the concentration of AZT. AZT increases the promoter activity of tartrate-resistant acid phosphatase (TRAP) and the binding and function of the nuclear transcription protein, NF-ƒÈB, in RAW264.7 cells. Therefore, the effect of AZT is mediated, at least in part, by enhancing RANKL-mediated osteoclastogenesis. Bone mineral density (BMD) in AZT-treated mice is decreased and histopathology shows marked osteopenia. These results support an important role of AZT-stimulated osteoclastogenesis in HAART-induced osteopenia.
INTRODUCTION
In AIDS therapy, inhibiting viral replication by various antiretroviral drugs is extremely important. Highly active antiretroviral therapy (HAART) has made a very significant advance in the treatment of AIDS, interrupting disease progression, keeping viral loads undetectable, enhancing recovery of CD4-positive lymphocytes, and significantly reducing mortality in HIVinfected
patients.1,2 Despite the clinical benefits of HAART therapy, it does not completely eradicate the virus. Therefore, patients with AIDS have to administer antiretroviral drugs over extended periods of time. As a result, a variety of metabolic complications associated with HAART have been reported. There are three major categories of HAART-associated metabolic diseases:
(1) nucleoside analogue-associated toxic effects including neuropathy, myopathy, pancreatitis, hepatic steatosis, and lactic acidosis;
(2) metabolic complications including redistribution of subcutaneous adipose tissue, insulin resistance, diabetes, and hyperlipidemia3,4; and (3) metabolic bone disease including osteopenia and osteoporosis.3-5
There have been many recent advances in our understanding of the cellular and molecular biology of bone remodeling and metabolic bone diseases. Bone remodeling depends on the tightly integrated activity of two major cell types: the osteoblasts, which make new bone, and the osteoclasts, which resorb old bone. Therefore, the balance between the number and activity of osteoclasts and osteoblasts is crucial in determining bone mass, which is directly related to bone fragility and fracture risk.6-9 Hormones, inflammatory cytokines, and growth factors are all involved in the regulation of osteoclast number, which depends collectively on rates of cell proliferation, differentiation, and death. There are two important molecules produced by osteoblasts/stromal cells that stimulate the differentiation of osteoclasts: macrophage colony-stimulating factor (M-CSF) and receptor-activator of nuclear factor k B ligand (RANKL). RANKL binds to RANK receptors on osteoclast precursors and triggers their differentiation into osteoclasts in the presence of M-CSF. Therefore, RANKL expression and subsequent signal transduction pathways play a crucial role in osteoclastogenesis. 6,9-11
It has been shown that the risk of bone complications is significantly higher in AIDS patients receiving HAART than in patients not receiving HAART.3,12-18 However, the association between HIV infection itself, HAART, and HIV-associated complications has not been fully elucidated. Paton et al. determined bone mineral density (BMD) in AIDS patients and found only minimal differences in BMD between HIV patients and controls.13 These results indicate that HIV infection itself does not induce bone loss. Tebas et al. measured BMD in HAART-treated AIDS patients and found that 50% of the patients had osteopenia, and 21% had osteoporosis.14,19 This incidence is significantly higher than in untreated patients or normal controls, suggesting there is a close connection between HAART and bone deficit. However, there are conflicting studies on the relationship of bone deficit to other HAART-induced metabolic
complications. Tebas et al. considered the bone deficit to be a unique HAART-induced metabolic complication, independent of lipodystrophy.19 Other studies suggest that HAART-induced decreased bone mineral density may be linked to fat redistribution.5
HAART most frequently employs three classes of anti-HIV compounds, including one or two HIV protease inhibitors (PIs) combined with two nucleoside reverse transcriptase inhibitors (NRTIs) or two NRTIs combined with one nonnucleoside reverse transcriptase inhibitor.20 Protease inhibitors, such as ritonavir, prevent cleavage and maturation of the proviral precursor, whereas nucleoside reverse transcriptase inhibitors, such as zidovudine (AZT), inhibit replication of the viral RNA genome.21-23 Although AZT has been widely used in AIDS therapy as a principal anti-HIV drug, whether AZT or other NRTIs can produce osteopenia/osteoporosis has not been definitively elucidated. Recently, Tsekes et al. surveyed the whole body compositions of 23 HIV-infected patients on AZT and other NRTIs using dual energy X-ray absorption (DEXA) and found that AZT and other NRTIs significantly decrease both body fat and BMD.24
In this study, we show that AZT increases osteoclast differentiation in vitro using a mouse macrophage preosteoclast cell line, RAW264.7, and mouse primary bone marrow macrophage-monocyte precursors. The mechanism of enhancement of osteoclastogenesis involves stimulation of the tartrate-resistant
acid phosphatase (TRAP) promoter, mediated through the transcription factor, NF- B, in a RANKL-dependent manner. Results were confirmed in AZT-treated mice that showed a significantly decreased BMD and histopathological features of severe osteopenia.
BMD is decreased in AZT-treated mice
To determine whether the stimulatory effects of AZT on osteoclast differentiation in cultured cells are also reflected in AZT-treated mice, an animal model was generated to investigate the effects of AZT on bone in vivo. Mice were administered 0.25 mg AZT orally per day for 4 weeks. The controls received
water without AZT. Subcranial BMD was measured by DEXA. The weights of the animals in both groups were identical at the end of treatment. The results in Figure 9 show that BMD in the AZT-treated mice was significantly decreased from 0.0462 +/- 0.0015 in controls to 0.0416 +/- 0.0001 (n =3, p <0.04).
Histological examination of tibias
To assess changes in bone structure, H&E staining of tibiae sections was performed (Fig. 10). Histology of bone sections demonstrated impressive histological features of osteoporosis characterized by increased trabecular separation and narrowed and fragmented trabeculae. The average trabecular separation in the AZT-treated group was increased to 202 +/- 3.9 um from 135 +/- 7.8 um in control animals (mean +/- SE, p <0.05). The reduced volume of the trabeculae in AZT-treated mice is consistent with the decreased BMD.
DISCUSSION
In the present studies, we examined the effects of AZT on osteoclast differentiation in vitro using a cultured mouse osteoclast
precursor cell line, RAW264.7, and primary mouse bone marrow macrophage-monocyte preosteoclasts. AZT accelerates the differentiation of osteoclast precursors in the presence of RANKL and increases the sensitivity of osteoclast precursors to RANKL. The effect of AZT is associated with increased activities of the TRAP promoter and the NF- B transcription factor. Furthermore, AZT-treated mice have decreased bone mineral density and osteopenia as determined by histopathology. To our knowledge, these studies are the first to demonstrate stimulatory effects of AZT on osteoclastogenesis in vitro and osteopenia in vivo. Our results are consistent with the clinical studies of Tsekes et al., who demonstrated that AZT and other NRTIs cause a reduction of bone mineral content in AIDS patients.24
24. Tsekes G, Chrysos G, Douskas G, et al.: Body composition changes in protease inhibitor-naive HIV-infected patients treated with two nucleoside reverse transcriptase inhibitors. HIV Med 2002;3: 85-90.
TRAP expression is increased during osteoclast differentiation.33 Differentiation of RAW264.7 cells into TRAP-positive osteoclasts generally requires 5-6 days of treatment with RANKL. However, AZT significantly accelerates the differentiation in cultured RAW264.7 cells. To optimize the stimulatory effect of AZT on osteoclast differentiation, we shortened the time of RANKL treatment and AZT stimulation to 2-3 days. This reduced time of stimulation results in a lower level of osteoclast formation in RAW264.7 cells treated with RANKL alone (Figs. 1, 2, and 5) than seen at 5-6 days of culture. This is also evident by comparing the larger (apprx 10-fold) effects of AZT in the RAW264.7 cell studies (Figs. 1, 2, and 5) to the apprx 2-fold stimulation using mouse osteoclast precursors that are incubated for 5 days (Figs. 3 and 4). Consistent with this, Feng et al. found that TRAP expression is undetectable or present at very low concentrations in osteoclast precursors and early-differentiated osteoclasts during the first 2-3 days of stimulation with RANKL.33 The shorter assay conditions maximized the observed effect of AZT on RANKL-stimulated osteoclastogenesis.
The occurrence of osteopenia and/or osteoporosis in AIDS patients receiving HAART has been reported in many clinical studies.34 However, most of the metabolic changes have been reported to be associated with PI-based regimens. Recently, switching from PI-based regimens to combined AZT and other NRTIs to avoid or reverse the metabolic complications observed during long-term PI-based antiretroviral therapy has been proposed.35 The results of a large-scale prospective randomized study and cohort studies indicate that switching to an NRTI-based regimen is not beneficial. Such a regimen not only increases
the risk of virological failure, but also does not uniformly improve metabolic and lipid abnormalities. However, these studies did not analyze the effects of NRTI-based regimens on the bone remodeling process. To examine the effect of NRTIs on osteoclast differentiation, we employed AZT, as it is the principal NRTI drug in various treatment regimens. There are conflicting reports on the effect of HAART on bone formation/resorption. A clinical survey of 112 male AIDS patients receiving PI compounds indicated that these compounds increased the incidence of osteopenia and osteoporo- (continued after graph)
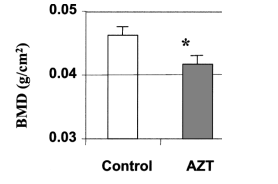
FIG. 9. AZT decreases BMD as measured by DEXA. Three male C57BL/6 mice aged 6 weeks received 0.25 mg AZT by a single daily oral feeding for 25 days. Three controls were given water without AZT. Bone mineral density (BMD) was measured by DEXA. Total bone mineral density is presented as the mean +/- SD in three pairs of mice. *p <0.05 in the t test.
sis.19 Jain and Lenhard compared the effects of various PI drugs on bone resorption and found that some PIs, but not all, increase bone resorption. The stimulatory effect on bone resorption appears to depend on the specific PI used.4 AIDS patients with advanced disease or with symptoms of wasting syndrome have been reported to be at high risk of developing osteopenia/osteoporosis.36 In contrast, Nolan et al.16 proposed that PI drugs may increase BMD based on studies of 131 PI-treated AIDS patients. These discrepant clinical conclusions are possibly due to differences in the selection criteria of AIDS patients and the complexity of various HAART regimens. Since HAART is a complicated therapy composed of up to 16 antiretroviral drugs and there are many therapeutic regimens, how different antiretroviral drugs interact with each other as well as
how they affect bone formation and resorption have not been studied. Although our studies demonstrate the stimulatory effect of AZT on osteoclastogenesis in vitro and in AZT-treated mice in vivo, they do not suggest that other NRTIs will have the same effects on bone. It will be necessary to examine the effects of other NRTI compounds, such as didanosine (ddI), stavudine (d4T), and lamivudine (3TC), alone and in combination, on bone resorption in vitro and in vivo. Further studies are needed to determine the effects of combined NRTI plus PI therapy on bone remodeling processes. The animal model used here should be very useful for such studies.
TRAP is one of the principal early markers of osteoclast differentiation. 6,37 AZT-stimulated osteoclastogenesis is mediated through activation of the TRAP promoter (Fig. 6) and requires RANKL (Figs. 1 and 3). These results prompted us to analyze the mechanisms of TRAP gene regulation during AZT-stimulated
osteoclastogenesis. The promoter region of the TRAP gene contains several transcription factor-binding sites including those for AP1, PU.1, and NF- B. We demonstrated that AZT increases the binding of the NF-k B transcription factor.
Consistent with this, we found that AZT stimulates the activity of luciferase driven by NF-k B binding confirming the involvement of NF-k B in AZT-stimulated osteoclastogenesis. It is known that NF-k B regulates RANKL-mediated osteoclast differentiation.6,31 When RANKL binds to RANK, intracellular signaling cascades in osteoclast precursors are activated involving TRAFs,38-40 NF- B,9,41,42 JNK/AP1,43 and the MAP kinase family.44 Mice lacking NF-k B show defective bone resorption activity and have severe osteopetrosis, indicating that NF-k B plays a crucial role in bone resorption by osteoclasts. 37,45-47
Bone biology studies to date have examined only the effects of PIs. Jain and Lenhard showed that some PIs (NFV and LPV) inhibit osteogenesis and expression of OPG leading to increased osteoclastogenesis and bone resorption.4 In contrast, Wang et al. demonstrated that ritonavir decreased nuclear translocation of NF-k B and inhibited the phosphorylation of Akt.48 They also demonstrated that ritonavir inhibits osteoclast differentiation and function by blocking RANKL induction of NF-k B. Although the signaling pathways involved in the stimulation of osteoclastogenesis by AZT have not been elucidated, our data
support the involvement of the RANK/RANKL pathway. AZT may act synergistically to enhance RANKL-mediated signaling pathways, because RTIs require coadministration with RANKL to stimulate differentiation of osteoclast precursors. AZT increases osteoclastogenesis in RAW264.7 cells and primary osteoclast precursors in vitro and induces low BMD and severe osteopenia in mice. These effects of AZT on osteoclastogenesis in vitro are observed at concentrations in the low micromolar range, not far from the blood levels of 0.18-0.78 uM reported for patients.49,50 Similarly the decreased BMD and osteoporosis is observed in mice receiving 10 mg/kg/day AZT, a dose equal to that commonly used in AIDS patients.49,50 Therefore, the effects of AZT on osteoclastogenesis in our experiments are likely to be pharmacologically relevant.
In conclusion, AZT stimulates the activity of the TRAP promoter and induces a dose-dependent increase in osteoclast differentiation. The effect of AZT is mediated at least in part by enhancing, dose dependently, RANKL-mediated osteoclastogenesis via stimulation of TRAP promoter activity and NF- B activation. AZT-treated mice show features of osteopenia including a low bone mineral density. These results indicate an important role for AZT-stimulated osteoclastogenesis in HAART-induced osteopenia and osteoporosis.
|
| |
|
 |
 |
|
|