 |
 |
 |
| |
Efficacy and Safety of Abacavir/Lamivudine Compared to Tenofovir/Emtricitabine in Combination with Once-Daily Lopinavir/Ritonavir through 48 Weeks in the HEAT Study
|
| |
| |
Reported by Jules Levin
CROI 2008, Boston
Kimberly Y. Smith1, Derek Fine2, Parul Patel3, Nicholaos C. Bellos4, Louis Sloan5, Phillip Lackey6, Denise H. Sutherland-Phillips3, Cindy Vavro3, Qiming Liao3, and Mark S. Shaefer3
1Rush University Medical Center, Chicago, IL, US; 2Johns Hopkins University School of Medicine, Baltimore, MD, US; 3GlaxoSmithKline, RTP, NC, US; 4Southwest Infectious Disease Associates, Dallas, TX, US; 5North Texas Infectious Disease Consultants, Dallas, TX, US; 6ID Consultants, Charlotte, NC, US
INTRODUCTION
Limited direct comparative data exist between the recommended dual NRTI fixed dose combinations, ABC/3TC and TDF/FTC.
The HEAT study is the first head-to-head trial to evaluate the efficacy and safety of these dual NRTI backbones with a boosted PI as part of recommended first-line treatments in HIV-1 infected subjects.
STUDY OBJECTIVES
To establish that ABC/3TC is virologically noninferior to TDF/FTC when administered in combination with LPV/r over 48 weeks.
To compare the safety and tolerability of ABC/3TC versus TDF/FTC over 96 weeks.
AUTHOR CONCLUSION
ABC/3TC is comparable to TDF/FTC in virologic efficacy when combined with LPV/r through 48 weeks.
Both treatment regimens were well tolerated with few discontinuations due to adverse events in either arm.
AUTHOR DISCSSION
ABC/3TC was virologically non-inferior to TDF/FTC through 48 weeks when each was combined with LPV/r.
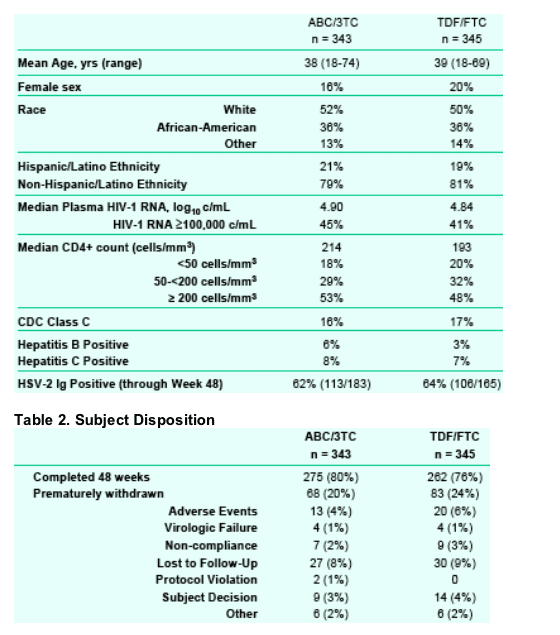
--68% vs. 67% of subjects (ABC/3TC vs. TDF/FTC) achieved an HIV-1 RNA <50 c/mL at Week 48 (ITT-E, M=F, switch included).
--75% vs. 71% of subjects (ABC/3TC vs. TDF/FTC) achieved an HIV-1 RNA <400 c/mL at Week 48 (ITT-E, M=F, switch included).
Efficacy results were consistent and robust to multiple analyses including when switches were counted as failures.
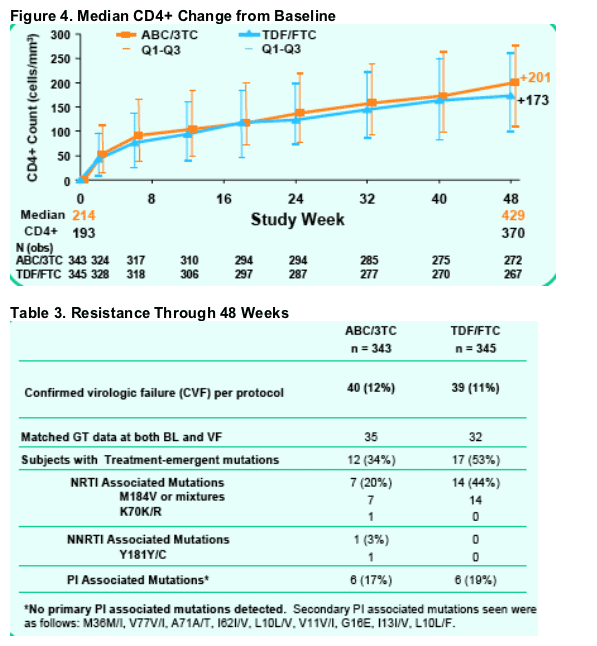
Median CD4+ cell responses differed between arms at Week 48 (429 cells/mm3 for ABC/3TC and 370 cells/mm3 for TDF/FTC).
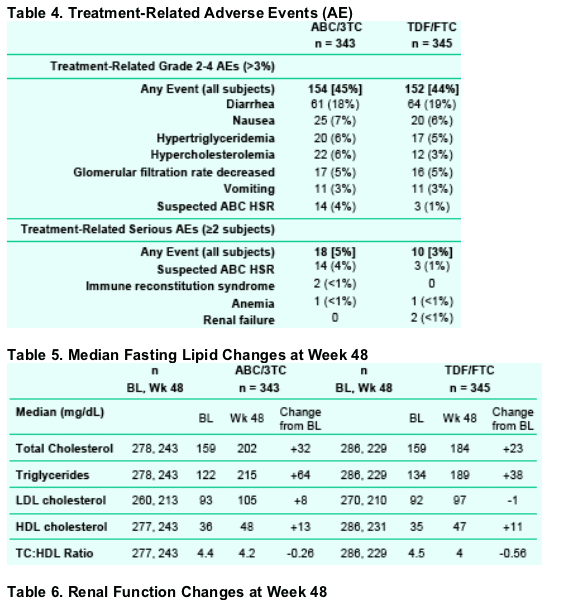
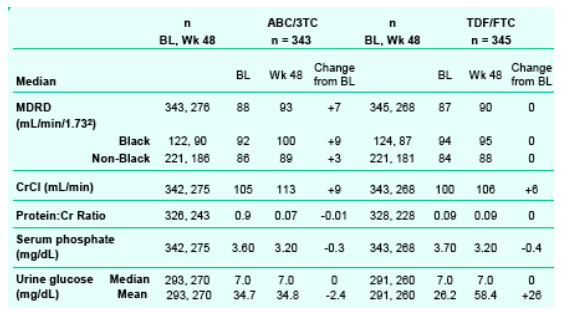
A conservative definition of virologic failure resulted in a somewhat elevated failure rate in both arms. Twice as many subjects receiving TDF/FTC failed therapy with an M184V or mixture which was a finding not previously reported.
No new safety findings were observed for either NRTI backbone.
--Rate of ABC HSR was 4% with ABC/3TC and 1% with TDF/FTC.
(HLA-B*5701 screening was not performed in this study)
--PRTD occurred in 0% with ABC/3TC and 1% with TDF/FTC.
--Lipids elevations were observed in both arms, however TC:HDL ratio remained below 5 for each.
A blinded analysis of the data was performed at Week 48 to protect the integrity of this 96 week study.
PROTOCOL DESIGNED STUDY FAILURE
Failure to achieve HIV-1 RNA <200 c/mL or confirmed rebound to ≥ 200 c/mL after confirmed reduction to <50 c/mL by Week 24.
Confirmed HIV-1 RNA ≥ 200 c/mL after Week 24.
Statistical Analysis
Non-inferiority of ABC/3TC compared to TDF/FTC would be established if the lower bound of the two-sided 95% CI on the difference in proportions achieving an HIV-1 RNA <50 c/mL at Week 48 was -0.12 or greater [ITT-E, missing=failure, switch included].
For stratified analyses by baseline HIV-1 RNA (< or ≥100,000 c/mL), the Cochran-Mantel-Haenszel method was used to compare virologic response between treatment groups.
Results
Table 1. Baseline Demographics and Characteristics
Other included pregnancy (1), site closure (2), and incarceration (3) in the ABC/3TC arm and site closure (1), death (1), incarceration (2) and relocation (2) in the TDF/FTC arm.
Protocol-Allowable Toxicity Switches
NRTI switches included suspected ABC HSR [14 (4%) ABC/3TC; 3 (1%) TDF/FTC] and Proximal Renal Tubule Dysfunction (PRTD) [0 ABC/3TC;
3 (1%) TDF/FTC].
-- PRTD definition: serum creatinine rise of ≥0.5 mg/dL from BL and serum phosphate < 2mg/dL or either of the former plus any 2 of the following: proteinuria ≥100 mg/dL, glycosuria ≥250 g/dL, low serum potassium <3 meQ/L, or low serum bicarbonate <19 meQ/L.
For PI-induced GI intolerability, LPV/r was permitted to be dosed twice daily. For treatment-limiting PI toxicity, LPV/r could be switched to FPV.
All subjects switched from LPV/r soft gel capsules (SGC) to LPV/r tablets at Week 48 to maintain formulation consistency during the study.
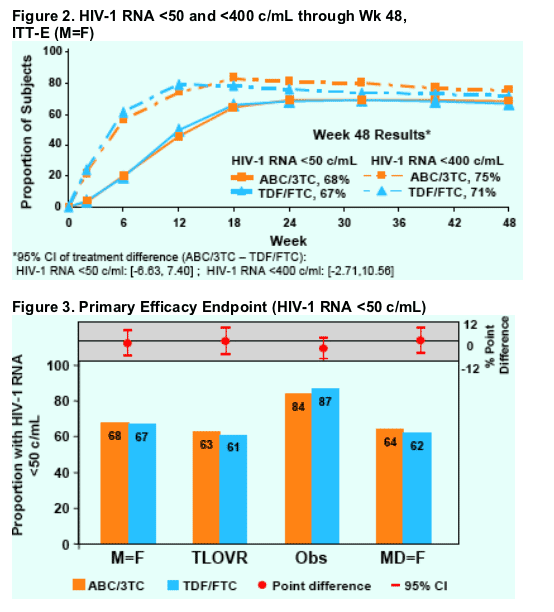
The lower bound of the 95% CI was greater than -0.12, thus establishing non-inferiority of ABC/3TC to TDF/FTC, (ITT-E, M=F, 95% CI (ABC/3TCTDF/ FTC) [-6.63,7.40]).
Consistent results were observed regardless of analysis method used including when switches were counted as failures (MD=F analysis).
In stratified analyses (ABC/3TC vs. TDF/FTC), 71% vs. 69% of subjects in the <100K viral strata achieved an HIV-1 RNA <50 c/mL; 63% vs. 65% of subjects in the ≥100K viral strata achieved this same endpoint at Week 48 (ITT -E, M=F).
Rate of virologic failure was similar in both groups.
The slightly higher rate of protocol-defined virologic failure may be attributed to the use of a more stringent criteria to determine success (HIV-1 RNA <200 c/mL).
|
| |
|
 |
 |
|
|