 |
 |
 |
| |
Tenofovir Disoproxil Fumarate (TDF) is Highly Active for Treatment of Chronic Hepatitis B (CHB) in Patients With Cirrhosis
|
| |
| |
Reported by Jules Levin
43rd Annual Meeting of the European Association for the Study of the Liver
April 23-27, 2008
Milan, Italy
M Buti,1 S Hadziyannis,2 P Mathurin,3 P Urbanek,4 M Sherman,5 S Strasser,6 C Wang,7 J Petersen,8 J Sorbel,9 E Mondou,9 J Anderson,9 and F Rousseau9
1Servicio de Medicina Interna Hepatologia, Hospital General Universitari Vall d'Hebron, Barcelona, Spain; 2Department of Medicine, Henry Dunant Hospital, Athens, Greece; 3Service d-Hepato-Gastroenterologie, Hopital Claude Huriez, CHRU Lille, Lille, France; 4Intern Klinika UVN Praha, Prague, Czech Republic; 5Toronto General Hospital, Toronto, Ontario, Canada; 6AW Morrow Gastroenterology & Liver Center, Royal Prince Alfred Hospital, Camperdown, NSW, Australia; 7Hepatitis Research Clinic, Harborview Medical Center, Seattle, Washington, USA; 8Klinik & Poliklinik F. Innere Medizin, Universitatsklinikum Hamburg-Eppendorf, Hamburg, Germany; 9Gilead Sciences, Inc., Foster City, CA and Durham, NC, USA
AUTHOR CONCLUSIONS
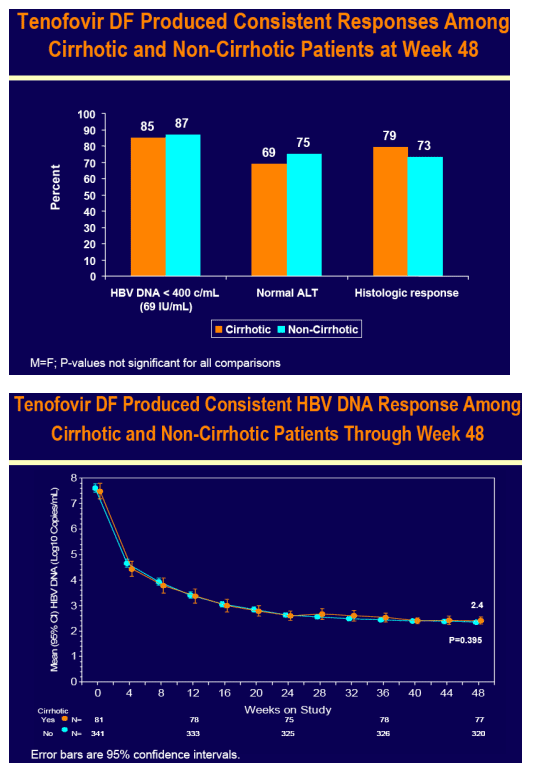
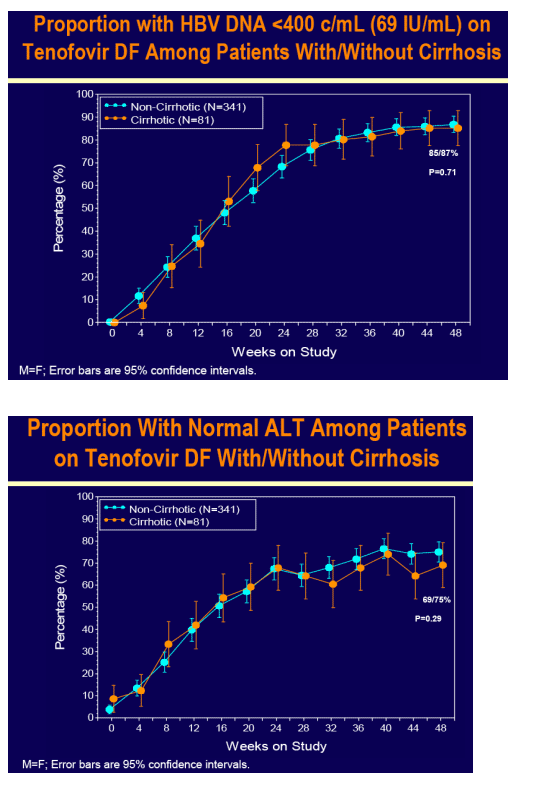
Tenofovir DF demonstrated potent HBV DNA suppression and good tolerability in patients with compensated cirrhosis due to chronic hepatitis B
Consistent efficacy responses were observed among cirrhotic and non-cirrhotic patients treated with tenofovir DF
AUTHOR SUMMARY
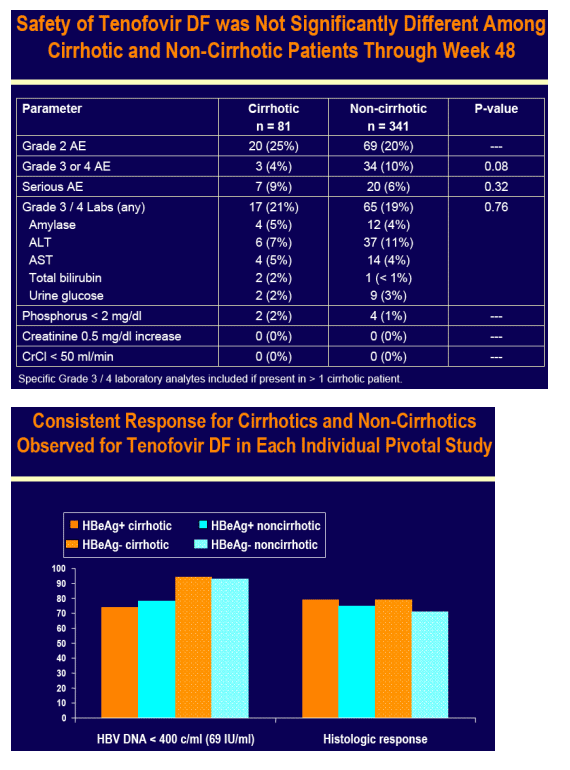
Tenofovir DF was well tolerated in cirrhotic and non-cirrhotic patients
No patient developed decompensated liver disease on tenofovir DF or adefovir dipivoxil
Resistance Surveillance
No resistance to tenofovir DF was detected at Week 48 in any patient
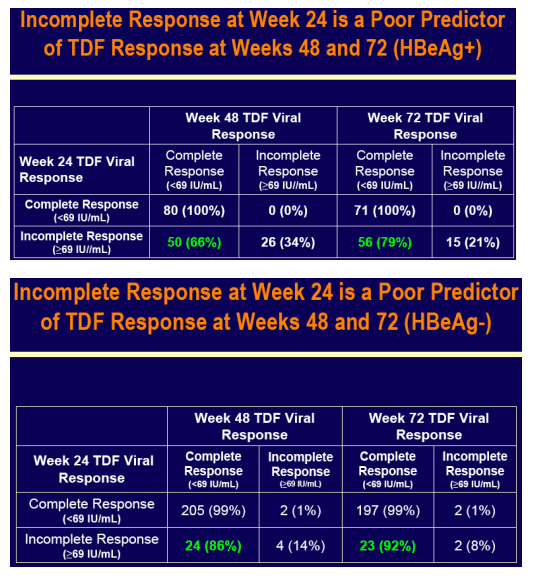
Across both pivotal studies 9 cirrhotic and 30 non-cirrhotic patients had HBV DNA > 400 c/mL (69 IU/mL) at Week 48, which included 10 (2 cirrhotic and 8 non-cirrhotic) patients with a 1 log10 increase in HBV DNA from nadir (7 of 10
were non-adherent)
Genotypic analysis was undertaken in all of these patients
- None had genotypic changes associated with resistance to tenofovir
INTRODUCTION
Tenofovir DF has shown superior efficacy to adefovir dipivoxil in treatment-na•ve patients with chronic hepatitis B in 2 pivotal studies
- After 48 weeks of tenofovir DF treatment 76% of HBeAg-positive patients and 93% of HBeAg-negative patients had HBV DNA <400 copies/mL (c/mL)
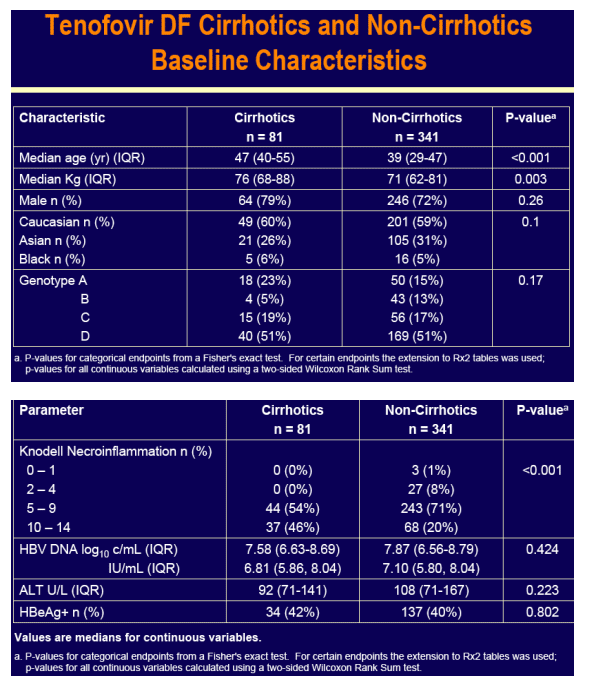
Both non-cirrhotic and cirrhotic patients enrolled in these pivotal studies
Objective
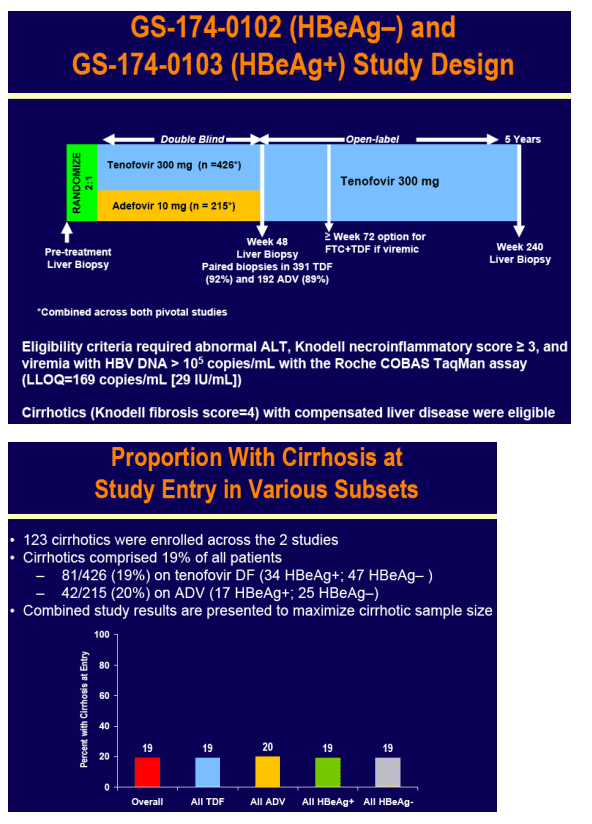
To evaluate the efficacy and safety of tenofovir DF among patients with cirrhosis due to hepatitis B participating in tenofovir DF pivotal studies GS-174-0102 (HBeAg-) and GS-174-0103 (HBeAg+)
Methods
Patients were randomized 2:1 to double-blind tenofovir DF (TDF) 300 mg or adefovir dipivoxil (ADV) 10 mg once daily for 48 weeks in studies GS-174-0102 (HBeAg-) and GS-174-0103 (HBeAg+)
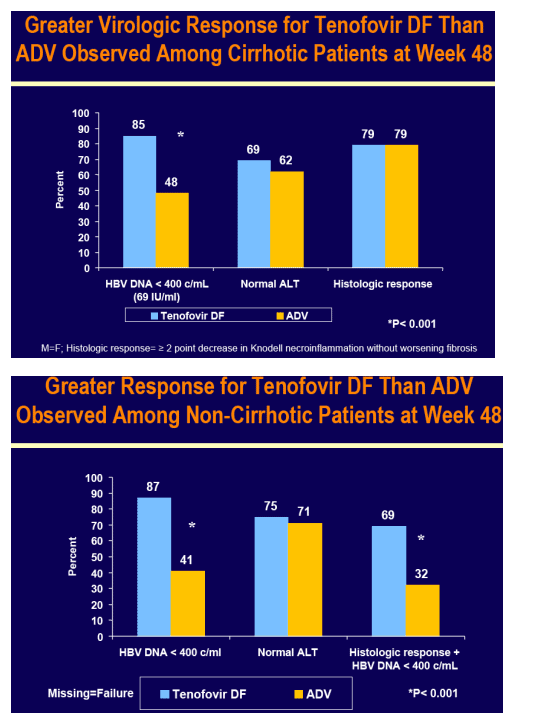
Virologic (HBV DNA < 400 c/mL [69 IU/mL]) and histologic response (≥ 2 point decrease in Knodell necroinflammation without worsening fibrosis) were prospectively evaluated
HBV DNA and safety laboratory parameters were performed every 4 weeks
For the subset analysis, the comparison of primary interest is tenofovir DF-treated cirrhotics versus noncirrhotics through Week 48 (ITT missing=failure [M=F])
|
| |
|
 |
 |
|
|