 |
 |
 |
| |
Sustained Viral Response Is Dependent On Baseline Characteristics In The Re-treatment Of Previous Interferon/Ribavirin Non-responders:
Final Results From The EPIC3 Program
|
| |
| |
Reported by Jules Levin
43rd EASL Conference
Milan, Italy
April 26, 2008
T. Poynard, M. Colombo, J. Bruix, E. Schiff, R. Terg, S. Flamm, R. Moreno-
Otero, F. Carrilho, W. Schmidt, T. Berg, E. J. Heathcote, P. Bedossa, P.
Mukhopadhyay, L. Griffel, M. Burroughs, C. Brass, J. Albrecht and the EPIC3
Study Group
Rationale for the Study, Unmet Medical Need
There is a growing unmet medical need for treatment options for previous treatment failures to IFN alfa + RBV combination therapy
By 2010, the majority of chronic hepatitis C patients will be either candidates for retreatment or patients who, for a variety of reasons, are not candidates for therapy
At the time the study was initiated, there were no approved therapies for patients who failed to respond to combination therapy, regardless of genotype.
Author Conclusions
Retreatment of previous treatment failures with significant fibrosis is effective
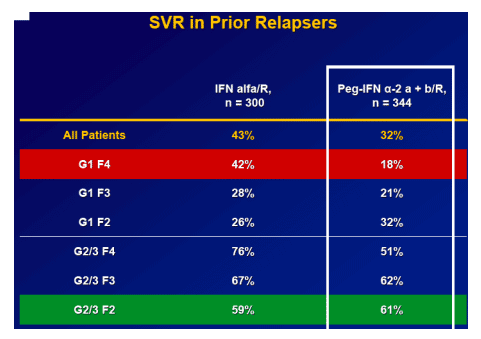
Overall SVR is 22%
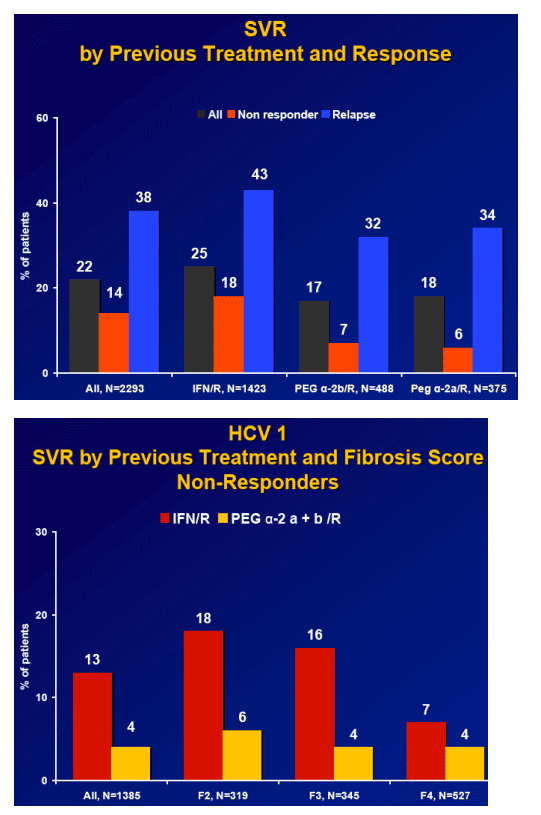
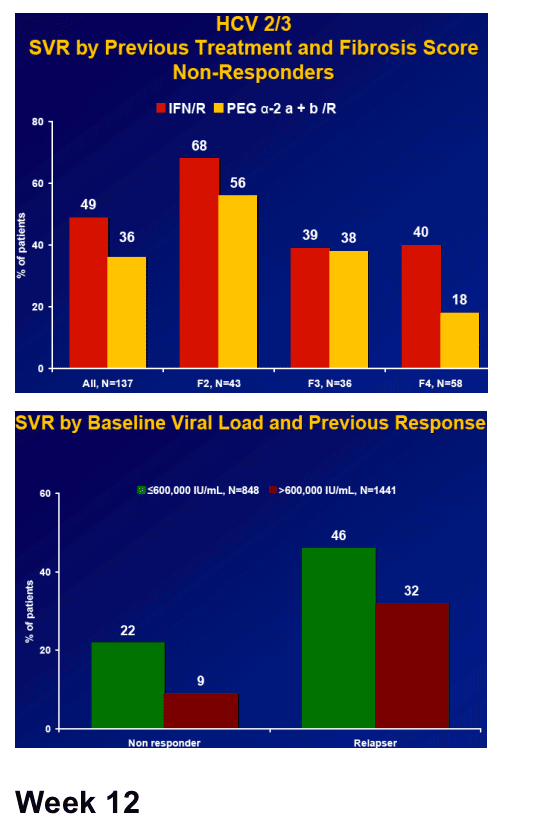
-- Non-responder SVR is 4-56%
-- Relapsers SVR is 18-62%
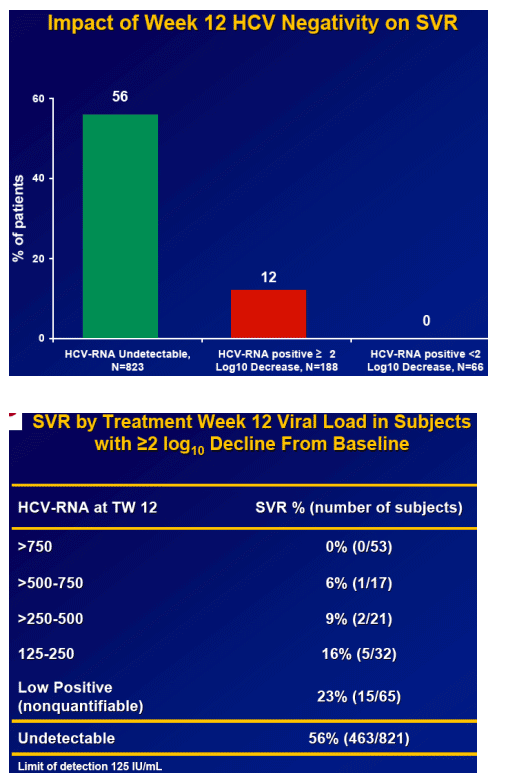
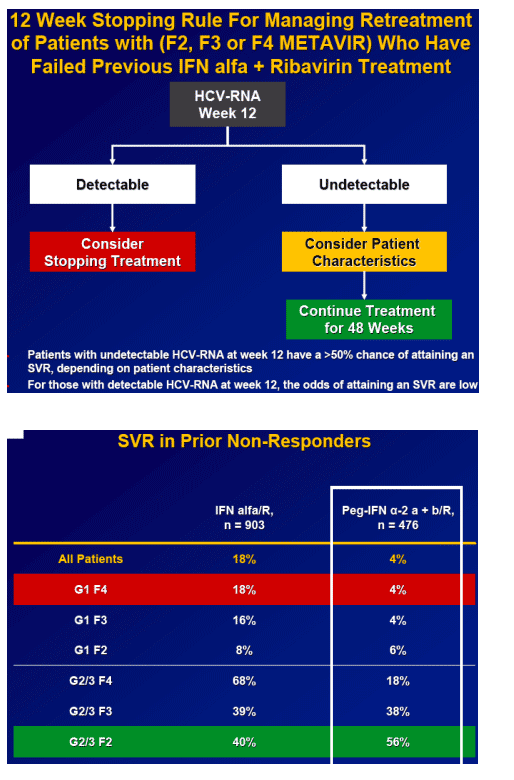
Week 12 HCV RNA is the best predictor of SVR.
-- 56% of those with undetectable HCV RNA at TW12 attained an SVR
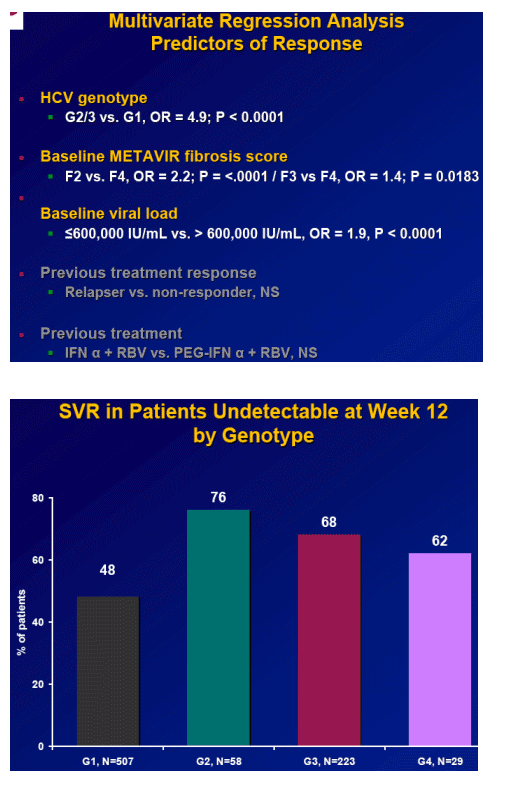
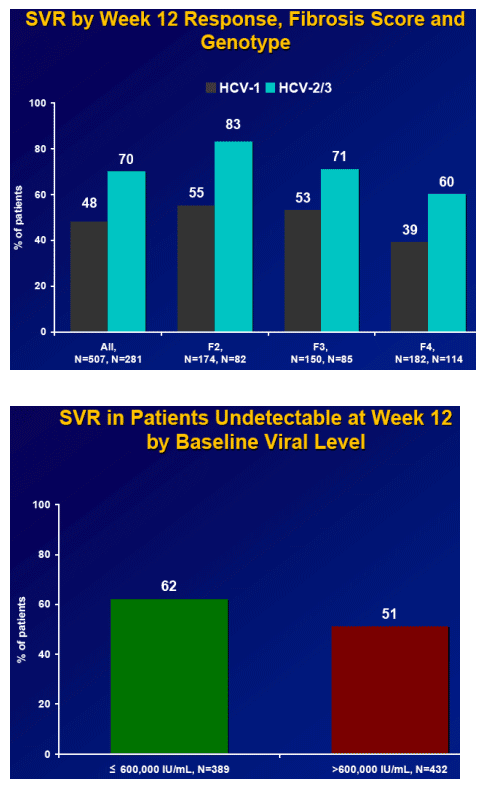
-- Genotype, fibrosis stage and baseline viral load remain significant predictors of SVR
ABSTRACT
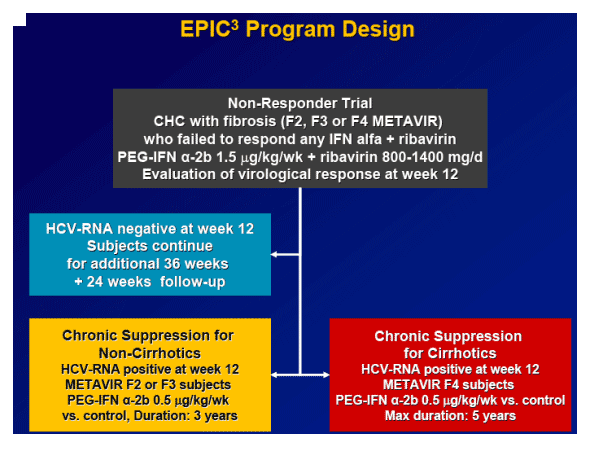
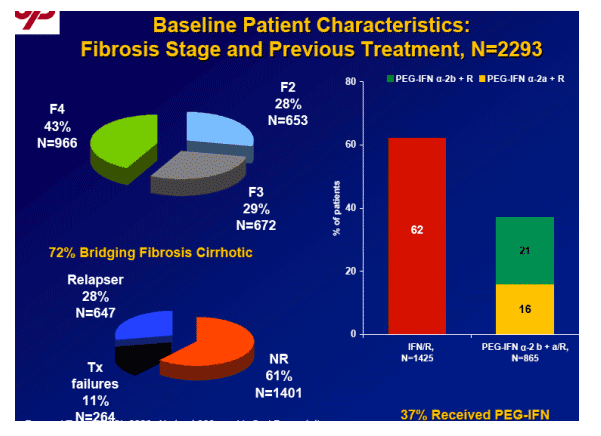
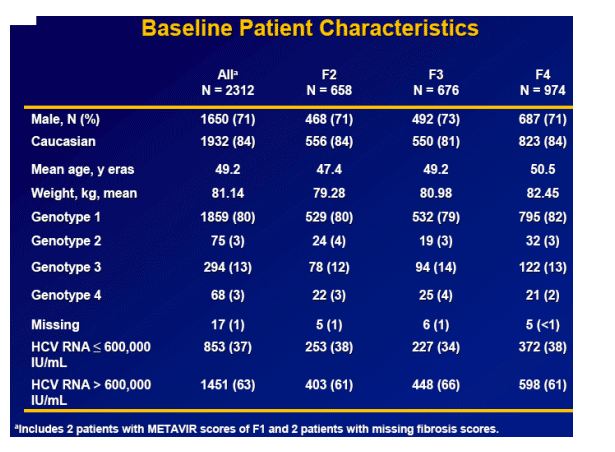
Background: EPIC3 includes a prospective trial assessing the safety and efficacy of retreatment of NRs with PegIntron/Rebetol (P/R). Aim: To determine SVR rates among Peg2a/2b (n=820) and alfa-interferon (n=1203) NRs retreated with P/R and to evaluate predictors of SVR.
Methods: NRs or those who relapsed after previous I/R treatment and had Metavir F2-F4 fibrosis received PegIntron 1.5 microgram/kg/wk plus Rebetol 800-1400 mg/d for up to 48 weeks. Plasma HCV-RNA was assessed at treatment weeks (TW)12, 24, and 48 and follow-up weeks 12 and 24 by quantitative Taq-Man assay (SPRI; sensitivity 125 IU/mL).
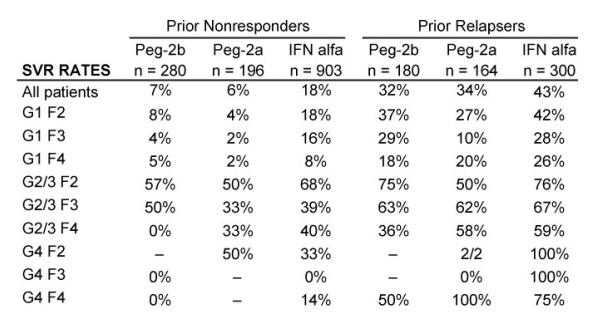
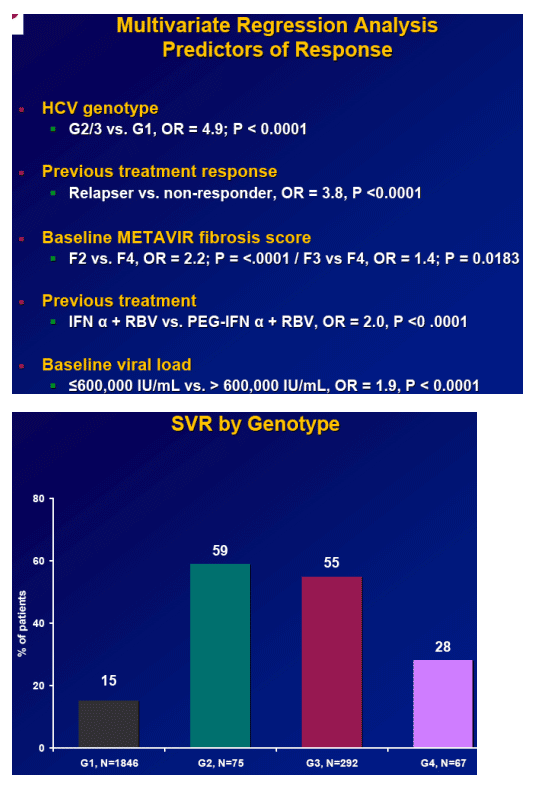
Results: Overall (full cohort) SVR rate was 22%. The likelihood of achieving SVR was greatly influenced by subjectÕs baseline characteristics. Generally, prior relapsers responded better than NRs (38% vs. 14%). However, genotype, degree of fibrosis, and prior treatment received were also important factors predictive of SVR; G2/3 subjects responded better than G1 subjects regardless of prior response (Table). Undetectable HCV-RNA at TW12 remained the most important predictor of SVR: 56% of subjects with undetectable HCV-RNA at TW12 achieved SVR vs 5% with a =2-log decrease in, yet detectable, HCV-RNA. Among subjects with undetectable HCV-RNA at TW12, the only significant predictors of SVR were genotype and fibrosis.
Conclusions: Genotype is the most predictive factor for SVR upon retreatment of subjects who failed previous treatment with I/R. G2/3 subjects respond better than G1 subjects, independent of prior treatment received and prior response. Across genotypes, fibrosis is also an important predictor of response.
Safety
Safety profile was similar to that previously reported for PEG-IFN alfa + ribavirin treatment and was similar among the fibrosis groups
Safety profile in cirrhotics (non-decompensated) was similar
|
| |
|
 |
 |
|
|