 |
 |
 |
| |
Results of a Safety, Tolerability and Pharmacokinetic Phase I Study of VCH-916, a Novel Polymerase Inhibitor for HCV, Following Single Ascending Doses in Healthy Volunteers
|
| |
| |
Reported by Jules Levin 43rd EASL
April 23-27, 2008
Milan, Italy
L. Proulx1, B. Bourgault1, N. Chauret1, R. Larouche2, M. Tanguay2, R. Thibert1
1ViroChem Pharma Inc., 275 Armand-Frappier, Laval (Quebec) Canada H7V 4A7; 2Anapharm - a PharmaNet company, 5160 Decarie, Montreal (Quebec) Canada H3X 2H9
AUTHOR CONCLUSIONS
VCH-916 was safe and well tolerated at up to 600 mg when administered as single oral doses.
The PK data indicated that the AUC, which was minimally affected by a high-fat meal, should achieve plasma concentrations necessary for anti-viral activity under fasted and fed conditions.
There were no major circulating metabolites (i.e. accounting for ≥ 10 % of the AUC of the parent compound) in the pooled plasma obtained following oral administration of 100 or 400 mg of VCH-916 to human subjects.
These results supported the initiation of a VCH-916 multiple-dose, 14-day study in treatment naive HCV-infected genotype 1 patients.
ABSTRACT
Background & Aim: The novel NS5B non-nucleoside polymerase inhibitor VCH-916 is being developed for the treatment of chronic HCV infection. The primary objective of the study was to evaluate the safety and tolerability of VCH-916 following oral administration in healthy volunteers. The secondary objectives were to determine the PK profile under fasting conditions and to evaluate food effect on bioavailability.
Methods: The study design was a randomized, sequential ascending single-dose (50, 100, 200, 400, 600 mg), double-blind, placebo-controlled trial (6:2 active to placebo) with a cross-over food effect arm (200 mg dose, high-fat meal). Safety monitoring included physical examination, vital signs, hematology, blood biochemistry, urinalysis, ECGs and adverse events (AEs).
Results: VCH-916 administration at doses between 50 and 600 mg did not result in any serious AEs. The most commonly reported AEs were throat irritations and headache. Additional AEs reported by at least 2 volunteers who received active treatment were: blurred vision, nausea, decreased blood pressure and somnolence. There were no clear increases of AEs observed in relation with increasing doses and 96% of all reported AEs were mild to moderate. No relevant changes from baseline in the laboratory results, ECGs or vital signs were observed among the active treatment groups. Under fasted condition, the extent of absorption (AUC) appeared proportional to the dose in the dose-range of 50 to 200 mg while it increased in a more than proportional manner over the dose-range of 200 to 600 mg.
The rate of absorption (Cmax) tended to increase in a proportional manner over the entire dose-range evaluated. Food did not have any effects on AUC but appeared to modestly decrease Cmax. VCH-916 displayed plasma exposure which could support twice daily dosing at doses ≥ 200 mg.
Conclusions: VCH-916 was safe and well tolerated up to 600 mg when administered as single oral doses. The PK data indicated that the compound's extent of absorption, which was not affected by food, should achieve plasma concentrations necessary for anti-viral activity under fasted conditions. These results supported the initiation of a multiple-dose, 14-day study in treatment naive HCV-infected genotype 1 subjects.
BACKGROUND
VCH-916 is a thiophene-2-carboxylic acid non-competitive allosteric inhibitor of HCV NS5B polymerase.
VCH-916 is a low micromolar inhibitor of HCV RNA dependent polymerase genotype 1a and 1b.
VCH-916 is equipotent against HCV genotype 1a and 1b replicons.
VCH-916 is selective for the HCV NS5B polymerase relative to the human
DNA polymerases alpha, beta, and gamma (IC50 > 100 uM).
The Therapeutic Index (CC50/EC50) in the replicon assay is > 400.
OBJECTIVE
Primary objective:
To evaluate the safety and tolerability of VCH-916 following oral administration of single ascending doses (from 50 to 600 mg) in healthy subjects.
Secondary objectives:
To determine the pharmacokinetic and metabolite profile of VCH-916 following oral administration of single ascending doses in healthy subjects under fasting conditions.
To evaluate the effect of food on the absorption of a 200 mg oral dose of VCH-916 administered under fasting conditions versus a high-fat meal.
PATIENTS & METHODS
Study Design:
- Double-blind, placebo-controlled, single dose-escalating study
- 6 active and 2 placebo subjects per group (doses: 50, 100, 200, 400, and 600 mg)
- 6 active and 2 placebo subjects for the food-effect group (dose: 200 mg, high-fat meal).
Safety Assessments:
- Physical examination, vital signs, hematology, blood biochemistry, urinalysis, ECGs and adverse events (AEs) assessments were performed throughout the study.
Pharmacokinetic and Metabolites Analysis:
- Serial blood samples were collected for up to 24 h and VCH-916 was analyzed using a validated LC/MS/MS assay.
- Presence and levels of circulating metabolites in plasma were analyzed using a LC/MS/MS assay.
RESULTS
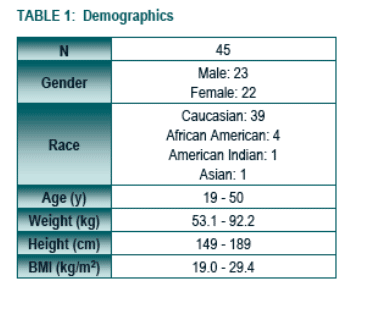
No notable differences were observed among treatment groups or between subjects who received active treatment versus placebo with respect to demographic parameters.
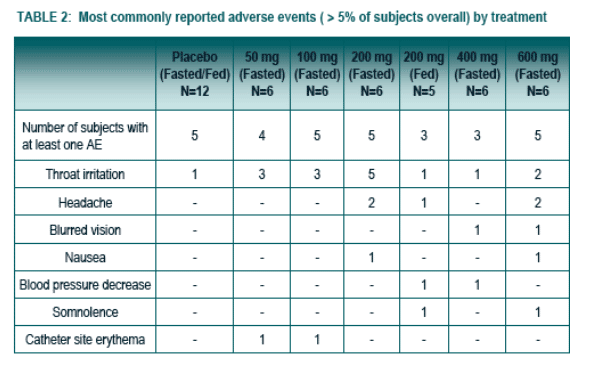
The most commonly reported AEs by more that one subject who received study medication and in a higher proportion than for subjects that received placebo are summarized in Table 2. All AEs were Mild to Moderate with the exception of 3 Severe AEs experienced by the same subject in the 600 mg dose (e.g., dyspnea, headache and nausea).
No Serious AEs or discontinuation were reported.
The frequency of AEs did not appear to be dose-related.
No clinically significant changes were observed with respect to vital signs, laboratory results and ECGs measurements.
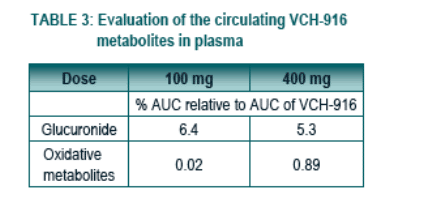
The metabolites observed following oral administration of 100 or 400 mg of VCH-916 are the glucuronide and oxidative metabolites accounting for 5-6% and 0.02-0.89% of the exposure of VCH-916, respectively.
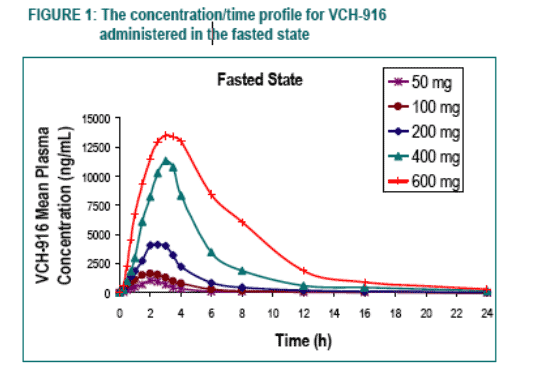
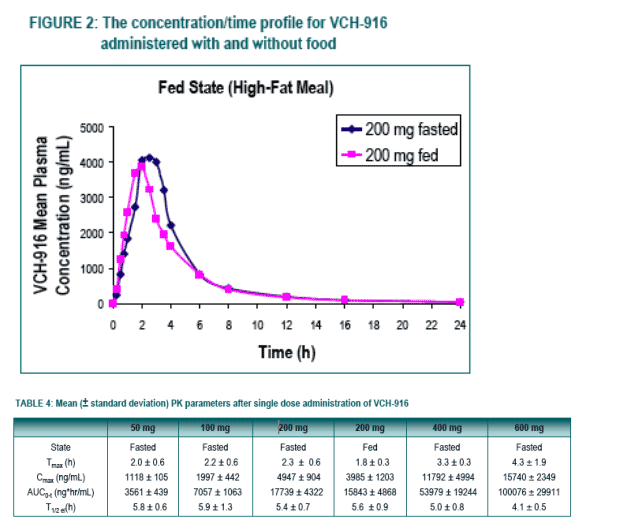
Under fasting conditions, the extent of absorption (AUC) appeared to be proportional to dose in the dose-range of 50 to 200 mg while increasing in a more than proportional manner over the dose-range of 200 to 600 mg. The rate of absorption (Cmax) tended to increase in a roughly proportional manner over the entire dose-range.
With regard to food-effect, the extent of absorption remained unaffected in the fed state when compared to the fasted state, however, there was a modest decrease in the rate of absorption when VCH-916 was administered with a high-fat meal.
|
| |
|
 |
 |
|
|